-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2019; 9(1): 434-438
doi:10.5923/j.ajmms.20190911.06

Tissue Factor and Platelet Microparticles in Acute Myocardial Infarction
Dina Ismail Badawy 1, Mona Hilmy Youssef Alrayes 2, Ahmed Yahiya Hegab 3, Reem Safwat 4
1Lecture of Clinical Pathology, Faculty of Medicine, Al-Azhar University, Egypt
2MD Professor and Head of Clinical Pathology Department Girls Faculty of Medicine, Al-Azhar Univesity, Egypt
3MD, Consultant Cardiology, National heart institute
4M.Sc., Specialist of Clinical pathology, National heart institute
Correspondence to: Dina Ismail Badawy , Lecture of Clinical Pathology, Faculty of Medicine, Al-Azhar University, Egypt.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

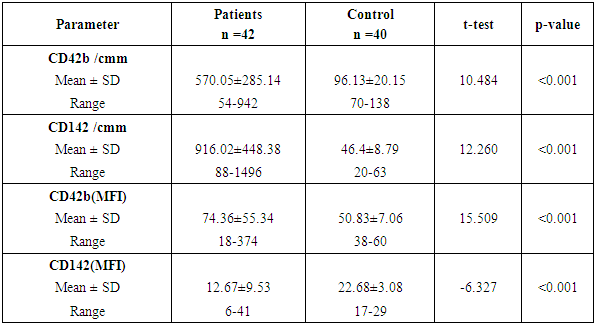
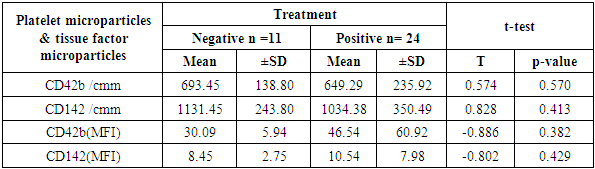
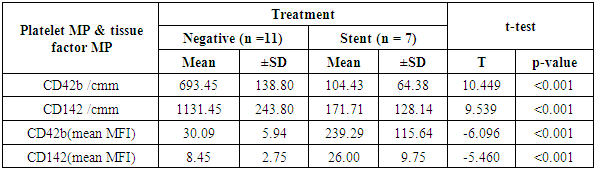
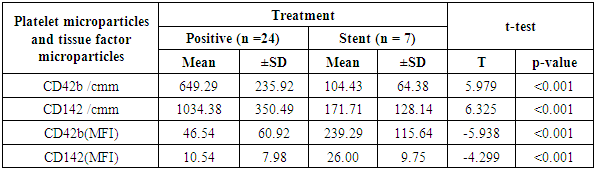
Aim of the work: This study aimed to assess the presence of tissue factor and platelet microparticles in cases of acute myocardial infarction in relation to clinical outcome. Patients and methods: This study included 42 patients with acute ST elevation Myocardial infarction. The counting of platelet microparticles carrying CD42b&CD42b (MFI and tissue factor microparticles carrying CD142&CD142 (MFI) was done by flow cytometry. Systemic blood samples of 40 healthy individuals (control) were obtained to assay the studied parameters. A comparison was done between the patients according to their clinical picture and mode of treatment used, and also between patients and control cases. Results: There was high statistical significant elevation of platelet microparticles (count and MFI) and tissue factor microparticles (count and MFI) in patients group in comparison with the control group. There was no statistically significant difference between patients on medical treatment and patients receiving no treatment as regard platelet microparticles and tissue factor microparticles. The study shows highly statistically significant difference between patients without and with primary PCI as regard platelet microparticles and tissue factor microparticles. The study also shows high significant positive correlation between platelet microparticles CD42b (MFI) and tissue factor microparticles CD142 (MFI), and shows non-significant correlation between platelet microparticles CD42b (MFI) and other parameters in control group.
Keywords: Microparticles, Acute myocardial infarction, Platelet, Tissue factor, STEMI
Cite this paper: Dina Ismail Badawy , Mona Hilmy Youssef Alrayes , Ahmed Yahiya Hegab , Reem Safwat , Tissue Factor and Platelet Microparticles in Acute Myocardial Infarction, American Journal of Medicine and Medical Sciences, Vol. 9 No. 1, 2019, pp. 434-438. doi: 10.5923/j.ajmms.20190911.06.
Article Outline
1. Introduction
- The main trigger of acute coronary syndrome (ACS) is usually a break in an atherosclerotic plaque which starts the clotting cascade activation. Atherosclerotic lesions with similar morphology may result in various forms of STEMI and NSTEMI events. The underlying mechanisms of this phenomenon remain elusive [1]. The overall pathogenesis starts upon the exposure of the sub-endothelial layer to the coagulation factors and platelets. The latter is the main player in the initiation and progression of ACS events via artery blockage and secretion of several pro-coagulant factors [2].Platelets secrete circulating microparticles (cMPs); small extracellular vesicles, released from circulating blood and vascular cells during damage or stress. These microparticles display cell surface proteins (CD) that indicate their cellular origin [3]. Plasma tissue factor (TF) is primarily stored in circulating microparticles (cMPs) [4]. Recently, blood samples from patients with acute myocardial infarction (AMI) showed significantly elevated concentrations of platelet derived cMPs (carrying CD 42 marker) and TF-carrying cMPs, relative to a control group of healthy subjects [5].Therefore, we conducted this study to assess the association between the blood levels of platelet-derived cMPs and TF-carrying cMPs and the clinical outcomes in patients with AMI.
2. Material and Methods
- SAMPLE COLLECTION AND PREPARATIONForty-two patients were admitted to Cardiac Care Unit (CCU) in the National Heart Institute. The study was carried out at the Immunology and Allergic Diseases Center, Al-Azhar University in collaboration with Cardiac care unit in National heart institute during the period between December 2014 to May 2015. We included patients with severe chest pain and ischemic ECG changes (ST segment elevation), troponin-positive and elevated CK-MB. The ages ranged between 28-71 years. We excluded patients with anemia, Infection, malignant disease, collagen disease, hyperthyroidism and cardiac disease other than AMI.Patients were classified according to treatment status into (1) Negative for medications in 11 patients, (2) Positive for medical treatment 24 patients, and (3) Patients applied stent by PCI7 patients. Patients were classified according to onset of chest pain into: (1) from 1-6 hours 12 patients, (2) from 7-12 hours 19 patients, and (3) from 13-24 hours 11 patients. Forty healthy volunteers were included in the study as a control group with no relevant history of medication within 7 days before sampling they were 37 males and 3 females.Ethical ConsiderationThe study takes into consideration the basic principles of biomedical ethics for the participants. Free and voluntary written informed consent was obtained. The participants were informed about their absolute right to be involved or to withdraw at any time from the study. Personnel privacy and confidentiality of the collected data was secured.METHODS: Flow-cytometry was conducted in Allergy and immunology Centre-AL-Azhar University on multi-color FACSCalibur (BD, Biosciences, San jose, USA). Cell Quest Pro software (BD Biosciences, San jose, USA) was used for data analysis. The optimal concentration for each dye used in flow was determined by titration experiments. Unstained samples were acquired to detect the sample auto-florescence. Mouse IgG2a FITC and IgG1 PE Controls (BD, Biosciences, San jose) were obtained for nonspecific binding detection. All dyes were applied gently to the Vortex before using to avoid any clumping. Filtered solutions were used to avoid any noise signals. Microparticles were stained with (20μL) (FITC)-conjugated monoclonal antibody (mouse anti-human Integrin CD42b) used for detection of platelet microparticles and 20μLPer Phycoerythrin( PE)-conjugated monoclonal antibody (mouse anti-human Coagulation Factor III/Tissue Factor anti-TF PerCP) used for detection of tissue factor expressing microparticles.Isotype control antibodies to set fluorescence threshold was included.The mixtures were incubated in the dark for 20 minutes at room temperature.Microparticles were re-suspended after addition of 200 uL sheath forward and side scatter were set at logarithmic gain. To identify cell marker and TF positive events, thresholds were set based on microparticles samples incubated with similar concentrations of Isotype control antibodies. MP-exposed antigen concentrations were calculated in each sample by multiplying the total concentration of positive MPs by the mean fluorescence intensity of the antigen exposure of the total positive MP population. complete blood picture with differential leukocyte count using fully automated cell counter (Sysmex Kx-21 N). Markers of cardiac enzymes as Troponin and total CK- MB using the Roche Hitachi 912 Chemistry auto analyzer and kits from Roche Diagnostic’s kits.STATISTICAL ANALYSISStatistical package for social science (IBM-SPSS), version 22 IBM Chicago USA will be used for statistical data analysis. Data will be expressed as standard deviation (SD) for quantitation data, number and percentage as described value for quantitative data. Student t-test was used to compare the mean between 2 groups. Chi-square test will be used to compare percentage of qualitative data. For all these tests, the level of significance (p. value) can be explained as: Non-significant p>0.05. Significant p<0.05 highly significant p<0.001.
3. Results
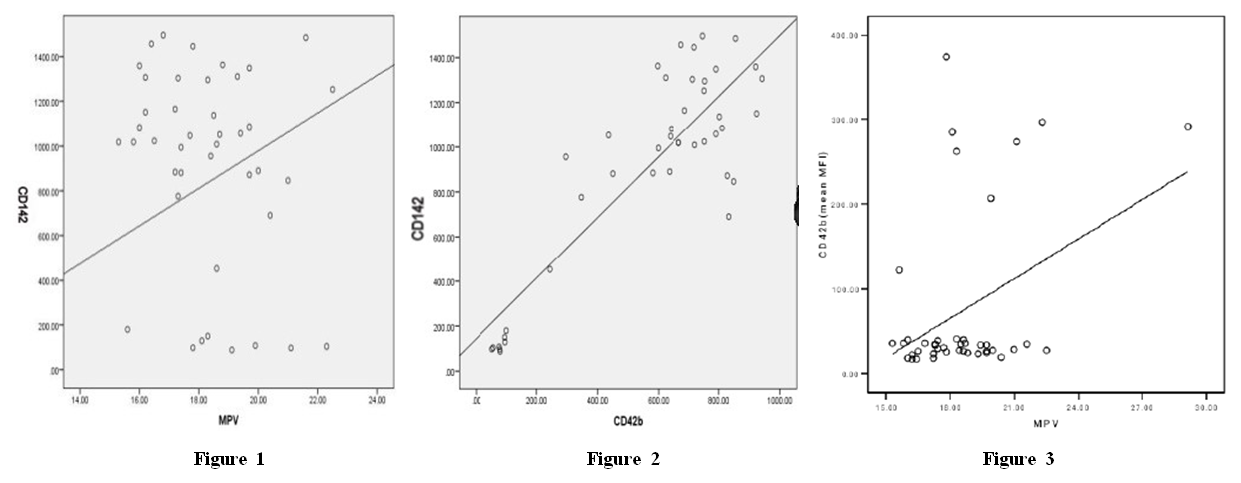
- CORRELATION STUDIESOur study revealed a positive significant correlation between MPV and CD142 (MFI) (R=0.338) (p=0.029) (figure 1) MP number showed significant positive correlation between CD142 and CD42b in patient (R=0.911) (p<0.001) and control group (R=0.366) (p=0.02) (figure 2).
 | Figure 1, Figure 2, Figure 3 |
|
|
|
|
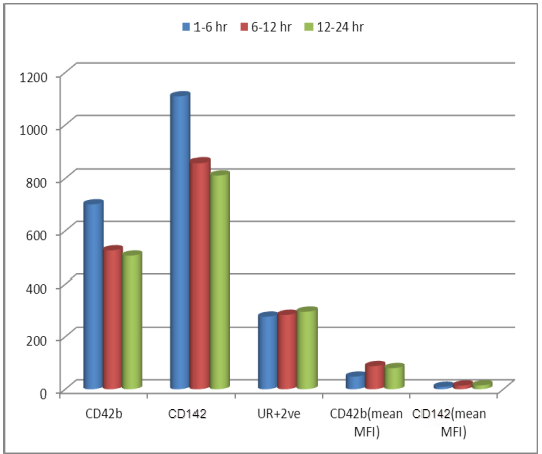 | Figure 4. Onset of pain according to platelet microparticles and tissue factor microparticles in patient group revealed no statistical difference of importance |
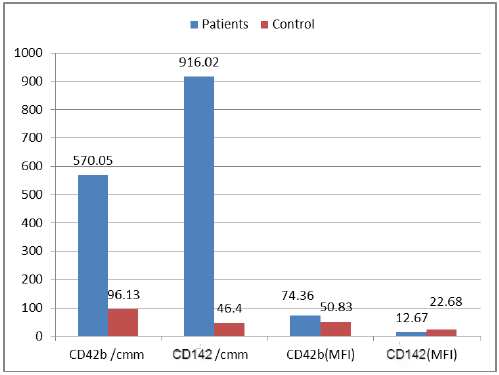 | Figure 5. Between patients and control according to platelets microparticles (count and MFI) and tissue factor microparticles (count and MFI) showed highly statistical of importance |
4. Discussion
- The present study showed significant elevations of the platelet distribution width and mean platelet volume in AMI patients, relative to control subjects (p <0.001). Similarly, Abrams et al [6] reported significant elevation of MPV and PDW in acute myocardial infarction group in comparison with both unstable angina and control groups. These findings highlight the possible diagnostic value of both parameters in AMI patients. Another study has measured the platelet volume indices in AMI and unstable angina patients; the authors found that PDW and MPV elevation are common features in ACS [7]. It was hypothesized that this association by stating that larger platelets usually contain higher levels of pro-aggregatory substances and adhesive receptors [8]. In another study by Yetkin et al [9]. the authors showed that platelet utilization during the pathogenesis of ACS triggers bone marrow megakaryocyte to produce larger platelets; hence, explaining the inverse correlation between platelet count and size. Our study revealed significant increases in platelet MPs (with CD42b marker) and MFI in the AMI relative to the control groups (p < 0.001). Similar data were provided by Ferroni et al. [10] who found that AMI cases have a significant elevation of peripheral blood platelet MPs than patients with unstable angina. This explains the occurrence of coronary artery thrombosis and myocardial necrosis in the earlier patients rather than the latter. Different reports showed that such elevation in MPs count emulates platelet activation in AMI cases. Platelet MPs induce blood coagulation through phosphatidyl serine in their surface layer and expression of TF [11].Tissue factor (expressing CD142) count and MFI showed a significant elevation in patients than control group (P< 0.001). Chirinos et al [12], in 2017, clearly demonstrated an increased MP count of different origins in patients with AMI when compared to the control subjects. They observed that the TF-bearing PMPs (CD42/CD142-positive) were significantly increased in both SA and AMI patients. These findings shed new light on the pathological mechanism of the pro-coagulant state in cardio-vascular patients. Wolf et al (2013) found that platelets, elevated MPs, endothelial cells and monocytes all are responsible for platelet activation in AMI patients. In addition, myocardial ischemia mainly results from the interference between platelets, endothelial cells and monocytes [13]. comparison between patients receiving medications and patients on no medications showed no statistical difference as regard PMP and TF+MP (P<0.05). On the other hand, there was significant reduction of PMP and TF+MP counts in patients with stent in comparison with both patients on no medications and patients receiving medications (P <0.001) Oppositely, PMP and TF+MP (MFI) were significantly elevated in in patients with stent in comparison with both patients on no medications and patients receiving medications (P<0.001). In the present work, AMI patients showed elevated ST-segment by ECG; some patients were with inferior lesion and some were with anterior lesion. Both groups showed no statistical difference in parameters (PMP and TF+MP). These results are in contrast with Tan et al. (2012) who stated that AMI patients have a significant increase in platelet MPs than unstable angina patients. The highest increase in platelets MPs are found in the STEMI group with significant difference relative to the NSTEMI group. In AMI cases, the platelet MPs are dependent on the spread of myocardial damage (peak CK-MB and peak SGOT) [14]. Our study revealed that MPV of the AMI patients was negatively correlated with the CD142+ TF+MP count (p= 0.02), while was positively correlated with the MFI of CD42b+ PMP and CD142+ TF+MP (p= 0.029).The CD42b+ PMP count was positively correlated with CD142 + TF+MP count (r= 0.86) and negatively correlated with the MFI of CD42b+ PMP and CD142+ TF+MP (r= 0.81). Further, Biasucci et al. (2012) studied variation in levels of MP in ACS. They found that endothelial tissue factor microparticles and PMP peak-levels (at day 1) were significantly correlated with peak of high sensitive CRP. They explained that; the relationship between MP shedding and high-CRP might be related to its interaction with complement. Activation of C5a has been shown to induce MP shedding. This is simply explained by 2 different pathways of a common acute reaction involving inflammation, coagulation and endothelium activation that concurs to initiation and persistence of coronary instability [15]. George et al (2015) studied TF+EMP and PMP levels in ACS and their relation to left ventricle function. They found that TF+ EMP as well as PMP showed direct correlation with ejection fraction. Its levels diminish as the LV function worsens. It is not known as to why MPs decrease in patients with LV dysfunction. They concluded that, the elevated levels of microparticles in ACS patients may reflect a protective effect in these patients [16].
5. Conclusions
- Platelet microparticles and tissue factor microparticles could be useful markers for diagnosis and prognosis of patients presented with STEMI. However, the MP levels considered physiological and pathological are still controversial.
ACKNOWLEDGEMENTS
- We want to express our sincere appreciation to Dr. Mona H. Alrayes, MD (Head of the Clinical Pathology Department, Al-Zahraa University hospital) for her support to our efforts during this work.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML