-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2019; 9(1): 427-429
doi:10.5923/j.ajmms.20190911.04

Significance of Polymorphism G1691A of the FV Gene to the Risk of the Occurrence of Thrombophilic Conditions in Chronic Myeloproliferative Diseases
Dildora Bakhramovna Shamsutdinova, Khamid Yakubovich Karimov, Kodirjon Tukhtabaevich Boboev
Department of Molecular Medicine and Cell Technologies, Scientific Research Institute of Hematology and Blood Transfusion of the Ministry of Health of the Republic of Uzbekistan, Tashkent, Uzbekistan
Correspondence to: Dildora Bakhramovna Shamsutdinova, Department of Molecular Medicine and Cell Technologies, Scientific Research Institute of Hematology and Blood Transfusion of the Ministry of Health of the Republic of Uzbekistan, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

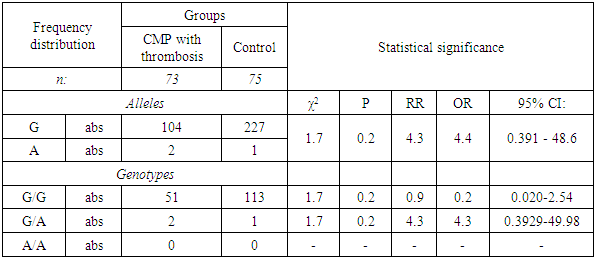
The aim of the study was to study the role of the FV gene mutation (G1691A) in the risk of developing thrombophilic conditions in chronic myeloproliferative diseases. The results indicate differences in the frequency of occurrence of the unfavorable allele A and the heterozygous genotype G/A of the FV gene polymorphism (G1691A) between the studied groups of patients and controls. In particular, the G/A genotype carriage was registered only in the group of patients with chronic myeloproliferative diseases (CMP) with the highest frequency among patients with thrombosis (χ2 = 1.7; P = 0.2; OR = 4.4; 95% CI 0.391 -48.6). In turn, these facts confirm the low diagnostic significance of the studied genetic marker in the development of thrombosis in chronic myeloid leukemia (CML) and polycythemia vera (PV), which emphasizes the need to study several genetic markers that predispose to thrombosis.
Keywords: Chronic myeloproliferative diseases (CMP), Chronic myeloid leukemia (CML), Polycythemia vera (PV), Carriage, Gene, Allele, Genotype
Cite this paper: Dildora Bakhramovna Shamsutdinova, Khamid Yakubovich Karimov, Kodirjon Tukhtabaevich Boboev, Significance of Polymorphism G1691A of the FV Gene to the Risk of the Occurrence of Thrombophilic Conditions in Chronic Myeloproliferative Diseases, American Journal of Medicine and Medical Sciences, Vol. 9 No. 1, 2019, pp. 427-429. doi: 10.5923/j.ajmms.20190911.04.
Article Outline
1. Introduction
- Currently, one of the urgent and important problems of modern hematology are vascular thrombosis [2,3]. The high probability and difficulty of treating thromboembolic complications in hematological diseases necessitate the timely diagnosis of factors or early prediction of the risk of developing thrombophilic complications in patients with hemoblastosis, in particular in chronic myeloproliferative diseases (CMP) [4-6].Recent studies have shown that important potential genetic risk factors for thrombosis are mutation of the FV gene (G1691A) [1,8]. Numerous studies on the role of the FV gene (G1691A) in susceptibility to the development of thrombosis have revealed the presence of this mutation in all populations of Europe and Asia, which predominate among people of European descent (5-10% vs. 2-3%) [7,9].In this regard, we conducted a study of the frequency of occurrence of the FV gene (G1691A) among patients with CMP and conditionally healthy individuals.
2. Main Body
2.1. The Purpose of Our Research
- Study of the role of the FV gene mutation (G1691A) in the risk of developing thrombophilic conditions in chronic myeloproliferative diseases.
2.2. Material and Methods of Study
- The work was performed on DNA samples isolated from the peripheral blood of patients with the most common CMP — chronic myeloid leukemia (CML, n = 32) and polycythemia vera (PV, n = 79), as well as the control group (n = 114). The collection of material for the study was carried out on the basis of the SRI H&BT MH RUz clinic among people of Uzbek nationality. All patients included in the study according to nosology were divided into 2 groups: Group 1 - persons with CML, Group 2 - with PV, each group being divided into two subgroups A - with thrombosis and B - without thrombosis.Genotyping of the samples and detection of the FV gene mutation (G1691A) was performed by PCR on an Applied Biosystems 2720 device (USA) using the reagents of Scientific Production Association (SPA) Liteсh (Moscow, Russia), according to the manufacturer's instructions. Statistical analysis of the results was carried out using the statistical software package "OpenEpi 2009".
2.3. Results of the Study
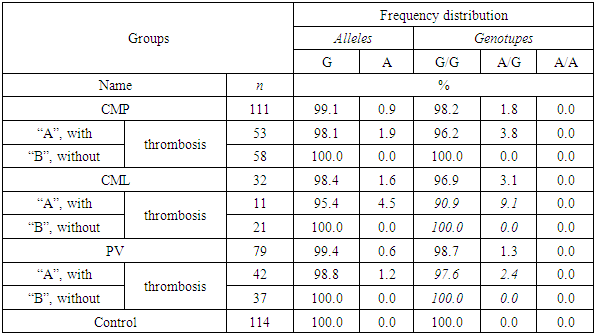
- The frequency of alleles of the polymorphism of the FV gene (G1691A) was characterized by the fact that in the main group of patients the proportion of allele G and A was 99.1% and 0.9%, respectively, in the control group their values were slightly different and amounted to 99, 6% and 0.4%, respectively. Analysis of the distribution of genotypes G / G and G/A in the main (98.2% and 1.8%) and control groups (99.1% and 0.9%) shows that the proportion of heterozygous genotype among patients is 2 times higher than in the control group (table. 1).
|
|
3. Conclusions
- Considering that we studied the role of the FV gene polymorphism (G1691A) in the development of thrombophilic conditions in patients with CMP, the results indicate differences in the incidence of the unfavorable allele A and the heterozygous genotype G/A of the FV gene polymorphism (G1691A) between the studied groups of patients and control. In particular, the carriage of the genotype G/A was recorded only in the group of patients with CPS with the highest frequency among patients with thrombosis (χ2 = 1.7; P = 0.2; OR = 4.4; 95% CI 0.391-48.6). In turn, these facts confirm the low diagnostic significance of the studied genetic marker in the development of thrombosis in CML and PV, which underlines the need to study several genetic markers that predispose to thrombosis.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML