Sh. Kh. Khashimov, Z. R. Khaybullina, T. M. Kabulov
Department of Endovisual Surgery, State Institution “Republican Specialized Scientific-Practical Medical Centre of Surgery Named after Academician V.Vakhidov”, Tashkent, Uzbekistan
Correspondence to: T. M. Kabulov, Department of Endovisual Surgery, State Institution “Republican Specialized Scientific-Practical Medical Centre of Surgery Named after Academician V.Vakhidov”, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
The effect of restrictive bariatric surgery - laparoscopic sleeve gastrectomy on the parameters of lipid metabolism and the distribution of subcutaneous and visceral fat - was studied. The decrease in %EBW and the increase in %EWL have been persistent for 36 months after LSG, proving its restrictive effect. During the first year after LSG, there was a progressive weight loss, when overweight declined 4.1 times, and the percentage of lost weight reached 81.5±4.1%; The BMI at this time was characterized by values of 34.9±1.4 kg/m. Weight loss was accompanied by a decrease in the area of fat in the subcutaneous and visceral depot, as well as the positive dynamics of a inflammation marker - CRP. The reduction in visceral fat and CRP indicates a normalization of pro-inflammatory status as a result of LSG. Analysis of lipid metabolism parameters at various periods after LSG showed that the decrease in triglyceride concentration occurred quickly (as early as on the 7th day), and the cholesterol metabolism parameters — much slower — only 12 months after LSG, an increase in HDL cholesterol occurred. Excessive visceral fat in patients with morbid obesity is accompanied by impaired carbohydrate and lipid metabolism. As a result of the restrictive effect of LSG, there was also a positive metabolic effect. The positive dynamics of %EWL was followed by a decrease in VFA, SFA, as well as an improvement in lipid metabolism parameters and a decrease in cardiometabolic risk due to a reduction of TG/HDL.
Keywords:
Obesity, Inflammation, Bariatric surgery, Carbohydrate and lipid profile, Laparoscopic sleeve gastrectomy
Cite this paper: Sh. Kh. Khashimov, Z. R. Khaybullina, T. M. Kabulov, Fat Depots Distribution and Lipid Metabolism Markers in Obese Patients after Restrictive Bariatric Intervention, American Journal of Medicine and Medical Sciences, Vol. 9 No. 1, 2019, pp. 421-426. doi: 10.5923/j.ajmms.20190911.03.
1. Introduction
The prevalence of obesity took an epidemic character, from 1986 to 2000, the number of people with morbid obesity (BMI> 40) increased 4 times, and those with BMI> 50 - 5 times (from 1: 2000 people to 1: 400). Having such a trend of weight gain will lead to the fact, that by 2048, the entire adult population of USA will be overweight or obese [1]. Bariatric surgery provides a weight reduction of up to 30% per year and is considered to be not only clinically but also economically the most effective treatment of obesity [2-3], besides surgery provides compensation for obesity-associated metabolic disorders and comorbidity. Main goals of surgical treatment are to influence the course of obesity-related diseases, improving patients' quality of life, reducing the risk of premature death. At present, theories about the mechanisms of metabolic efficiency of bariatric operations have been revised. A decrease in body weight is combined with a significant positive effect on the main types of metabolism, contributing to reduce the frequency of the fundamental components of the metabolic syndrome [4]. While bariatric surgery was previously recommended to patients with a BMI of more than 40 kg/m2, in the standards of the ADA (American Diabetes Association for the treatment of DM2) of 2010, it was noted that bariatric surgery may be recommended for adults with a BMI> 35 kg/m2, suffering from type 2 DM, especially when symptoms or associated comorbidities are poorly controlled with lifestyle change and pharmacotherapy [5]. It has been proven, that the hypoglycemic effect of surgery develops immediately from the first day after the operation and is not associated with a decrease in body weight [6]. Meanwhile, features of lipid metabolism in the dynamics of bariatric interventions are not completely investigated.
2. Aim of the Study
The aim of this paper was to elucidate the effect of restrictive bariatric surgery – laparoscopic sleeve gastrectomy – on lipid metabolism markers and distribution of subcutaneous and visceral fat.
3. Materials and Methods
Data source and selection criteriaData were obtained from the database of Endovisual Surgery department of the State Institution “RSSPMC of Surgery named after acad. V.Vakhidov” for the period from 2015-2019. Criteria for inclusion of patients were: age from 18 to 60 years; BMI over 40 kg/m2; BMI 30-35 kg/m2 in the presence of at least one of the comorbidities (DM type II, obstructive sleep apnea, hypertension, dyslipidemia). Exclusion criteria were: age under 18 and over 60 years; ASA risk 4 and 5; chronic diseases of the digestive tract in the acute stage; history of alcohol or drug addiction; mental illness, which is a contraindication to the planned surgical interventions. The average amount of excessive weight was calculated as the difference between the actual weight and “ideal” weight of the patient, at which patients BMI will be equal to 29.9 kg/m2, according to the Metropolitan table. Patient demographics, diagnosis variables and treatmentThe study included 40 patients with morbid obesity, who were admitted to our department. 36 of them were females and 4 males. Concentration of C-reactive protein (CRP), blood lipid profile: total cholesterol (CH), triglycerides (TG), high density lipoprotein cholesterol (HDL), very low density lipoprotein cholesterol (VLDL) were determined on an automatic biochemical analyzer "VITROS-350" by “Ortho Clinical Diagnostics” (USA). The atherogenic index (AI) was calculated using the Klimov’s formula: AI = (CH - HDL)/HDL. Excessive weight loss (% EWL) was calculated by the formula: EWL% = 100% * (lost weight/(initial body weight - ideal body weight)). Multislice computed tomography (MSCT) was performed to assess the distribution of visceral and subcutaneous fat. The study was carried out on the Aquillion one 640 Genesis 640-slice computed tomography (Canon, Japan): at the level of the intervertebral L4-L5 disk in the craniocaudal direction: in the transverse image, adipose tissue was determined in aninterval with a density of from -50 to -160 HU. The following parameters were used: slice thickness — 0.5 mm, image matrix — 512x512, tube voltage — 120 kV, electric current— 100 mAs. Quantifying the abdominal fat depot included determining the total fat area in the area of interest (TFA), visceral fat area (VFA), subcutaneous fat area (SFA), calculation the VFA/TFA ratio. Signs of visceral obesity considered to be the area VFA more than 130 cm² [7].Laparoscopic sleeve gastrectomy (LSG) was performed on a multifunctional endosurgical complex, using controlled coagulation technologies with the LigaSure (MedtronicCovidien) and Ultra Cision Harmonic Scalpel (EthiconEndo-Surgery, Johnson & Johnson).Statistical analysisStatistical processing of quantitative data was performed using the mean (M), standard deviation (SD), standard error of the mean (m) with a normal distribution of the trait; in the case of an asymmetric binomial distribution – the median (Me), quartiles (Q25; Q75); To assess the statistical significance of differences, the Student’s t-test was used (with equal variances) and the Fisher’s – F test in other cases. Differences were considered statistically significant (reliable) at p <0,05 for all types of statistical analysis.
4. Results and Discussion
ResultsInitially, before the operation, all patients had excessive body weight (% EBW), which was 69,9±5,8% (Me = 65,3; Q25;75 = 27,2; 74,6%); excessive weight loss ( % EWL), occurred after LSG, was most intense in the first year after the intervention. The decline in% EBW and the increase in% EWL were persistent for 36 months after LSG, proving its restrictive effect (Fig. 1).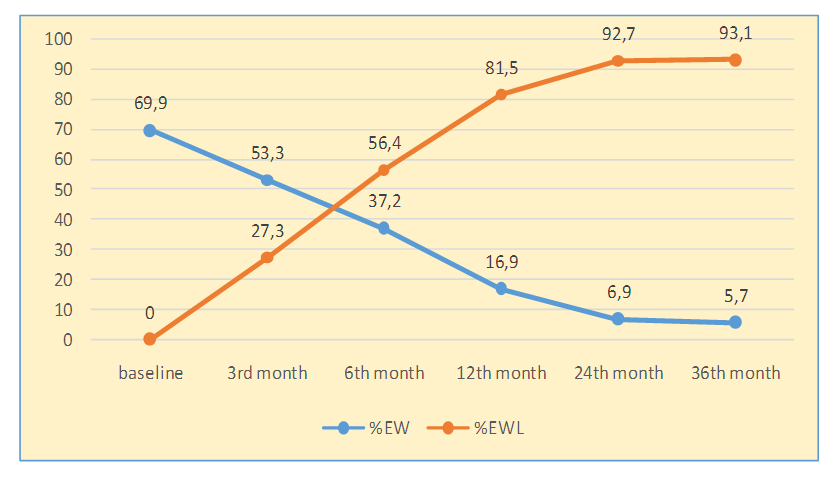 | Figure 1. The effectiveness of LSG in aspects of weight loss |
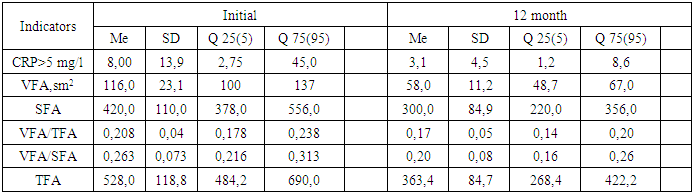
Line table illustrates, during the first year after LSG, there was a progressive loss of weight, when excessive weight declined 4.1 times, and the percentage of lost mass reached 81.5±4.1%; the BMI at this period was characterized by values of 34.9±1.4 kg/m2. It draws attention that the dynamics of all indicators of body weight was the most intense during the first 6 months, so; % EWL increased by 2.1 times, and the amount of excessive weight decreased by 2.7 times from the initial data. BMI at this time decreased by 1.3 times from the baseline. In the 2nd year of observation, the indicated weight loss trend continued, and in the 3rd year, it was characterized by stability in the retention of reduced weight, which can be assessed as a good result of LSG.Weight loss was accompanied by a reduction of fat area in the subcutaneous and visceral depot, as well as the positive dynamics of the inflammation marker CRP (Table 1).Table 1. Dynamical change of fat in depots after LSG
 |
| |
|
The table 1 presents, in 12 months after LSG, the TFA in the area of interest - diminished 1.6 times, the content of VFA and SFA narrowed 2.1 and 1.6 times, respectively. The number of patients in VFA/SFA has also changed over 0.4 - it has declined by 2.7 times. It should be noted, that the reduction in the area of visceral fat depot was more intense, relatively subcutaneous one, which determined a decrease in CRP by 3.5 times. It is known, that stimulant for the CRP synthesis in the liver is interleukin-6, actively secreted by visceral adipocytes and macrophages infiltrating adipose tissue [8]. The decrease in visceral fat and CRP specifies a normalization of pro-inflammatory status as a result of LSG. Of the interest, the distribution of patients by VFA in the area of interest before and after LSG, because initially, before the operation, VFA of our patients had a very wide range of fluctuations - from 87 sm2 to 166 sm2, while the level above 130 sm2 (critical for ascertaining visceral obesity) observed in 14 (35%) patients, and after LSG, the VFA value did not exceed 80 sm2 (Fig.2).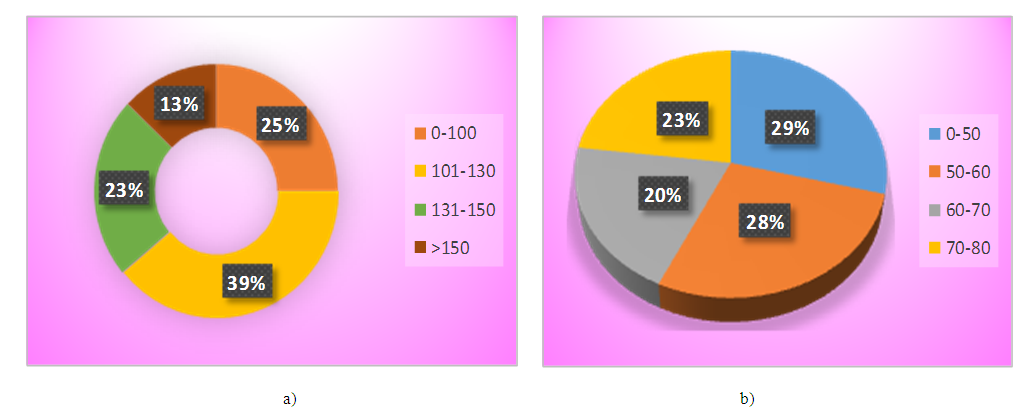 | Figure 2. a) Distribution of patients by VFA value (sm2), before LSG, b) Distribution of patients by VFA value (sm2), in 12th month of LSG |
Analysis of lipid metabolism parameters at various times after LSG showed that the decrease in TG concentration occurred quickly (as early as on the 7th day), and CH metabolism parameters — much slower — only 12 months after LSG, an increase in HDL cholesterol occurred (Table 2).Table 2. Blood lipid profile in 7th day after LSG
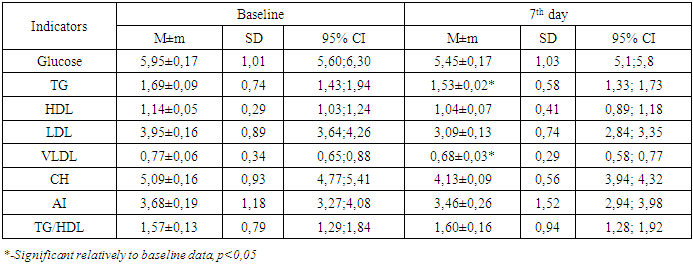 |
| |
|
According to the table, on day 7 there was a significant decrease in the concentration of TG and VLDL, there was a tendency to decrease of total CH, which affected the AI value, however, it has not changed statistically significantly (p> 0.05) relative to the initial data. Analysis of the lipid spectrum parameters in 3 - 6 months after LSG demonstrated that there was a tendency to a steady decrease in TG and total CH (p <0.05) (Table 3).Table 3. Blood lipid profile in 3 and 6months after LSG
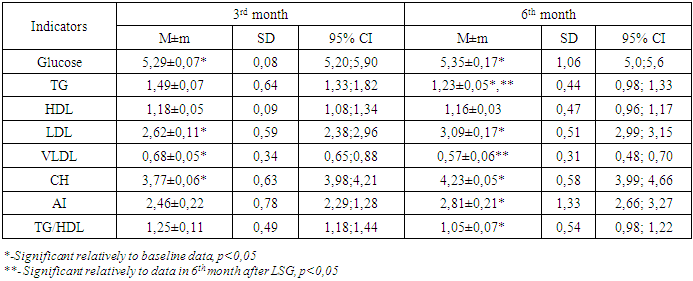 |
| |
|
As can be seen from the table, a gradual progressive decrease in TG is observed at each observation period after LSG, while by the 6thmonth after the operation all studied parameters (Glucose, TG, CH, VLDL, LDL, AI), with exception of HDL, are significantly different from the baseline. Attention is drawn to a significant decrease in the AI and a decrease in the TG/HDL ratio characterizing cardiovascular risk [9,10]. Hereby, 6 months after LSG, there is a significant improvement in lipid and carbohydrate metabolism.The following criteria were used, in evaluating the long-term results of surgery: by recovery of dyslipidemia, we implied the normalization of TG values below the 1.7 mmol/l and the increase in HDL over 1.3/1.03 mmol/l in females/males. Analysis of CRP, TG and HDL levels in visceral and subcutaneous fat depot, in the long-term period after LSG showed that hypertriglyceridemia remained in 5% of cases, dyslipidemia - in 15%, elevated CRP was in 3 (7.5%) patients (Table 4.).Table 4. Characteristics of fat depots and lipid profile units in distant periods after LSG
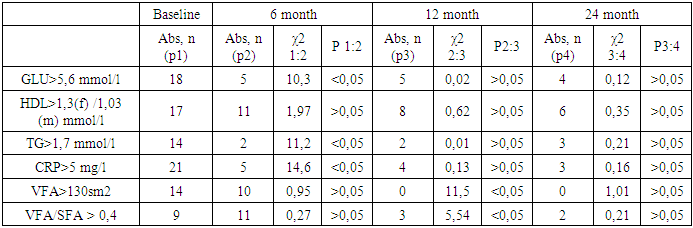 |
| |
|
As our observations have shown, an excessive visceral fat in patients with morbid obesity (MO) is accompanied by compromised carbohydrate and lipid metabolism. These results are consistent with reference data, where it is shown that with a BMI of more than 40 kg/m2, the concentration of C-peptide (a marker for the synthesis of endogenous insulin) increases by 2 times, the concentration of insulin - by 6 times, and HOMA-IR - by 10 times [11], which proves the formation of insulin resistance. As causes of insulin resistance are considered dysregulationof adipocytokines (increased adiponectin, reduced insulin sensitivity and decreased visfatin,which maintains insulin sensitivity), inflammation, infiltration of adipose tissue by macrophages [12-14]. Another pathogeneticway of hyperglycemia and hypertriglyceridemia,in subjects with MO, is adipsin resistance. Adipsin is a stimulator of hunger center; it is produced in the process of lipolysis and, along with acetylation stimulating protein, enhances glucose transport to adipocytes, where it also stimulates TG synthesis, therebyregulating serum TG levels and, partly, level of glucose [2]. With moderate visceral fat, an increased level of adipsin compensatory normalizes serum concentrations of glucose and TG, however, with a significant increase in VFA, current effect disappears due to violation of auto- and paracrine regulation of adipocytes in the synthesis of adipsin and other cytokines [6]. Probably, exactly given facts explain hyperglycemia more than 5.6 mmol/l and hypertriglyceridemia more than 1.7 mmol/l, which initiallydetectedin 45% and 35% of our patients, respectively. The dysregulation of adipocytokine secretion was combined with proinflammatory activity, as evidenced by an increase in CRP, the synthesis of which is induced by IL-6. Statistically, approximately 30% of the total circulating IL-6 originates from adipose tissue [12,15]. The decrease of HDL level in 57.5% of patients we examined was probably due to abnormalities of cholesterol metabolism and its transporter lipoproteins in the liver, as well as genetic factors.Because of the restrictive effect of LSG, we have obtained also a positive metabolic effect. Our results showed that the positive dynamics of %EWL was accompanied by a decline in VFA, SFA, as well as a refinementof lipid metabolism parameters and a decrease in cardiometabolic risk,in view of the fact that TG/HDL ratio is reduced.Hypertriglyceridemia and HDL reduction are known to be the most significant prognostic markers and predictors of cardiovascular risk [14]; Hypertriglyceridemia combined with an increase in waist circumference is called “hypertriglyceridemic waist” and is the first sign in identifying a high risk group for developing coronary heart disease and the TG/HDL ratio determines cardiometabolic risk [13].It is commonly accepted, that bariatric surgery significantly, by 29-40%, reduces the overall mortality in patients with obesity in comparisonwith patients treated with diet and drug (HR = 2.02; 95% CI, 1.63-2.52) [5]. According to the results of a large retrospective study of 8385 patients (in 43% conducted LSG, in 17% gastric shunting, and in 40% adjustable gastric banding) a study of 5-year survival after bariatric operations demonstrated, mortality during 4.3 years of follow-up was 1.3% - 105 cases. An interesting fact is that, out of 25155 obesity patients who did not undergo surgery, this figure was 2.3% (583 cases) [5]. This convincingly proves the effectiveness of bariatric operations in general and LSG in particular.
5. Conclusions
1. The restrictive effect of LSG is maximum in 12 months after the intervention, since it provides a loss of weight (% EWL) = 81.5% and is stable for 3 years after surgery, where % EWL is at least 94%.2. 12 months after LSG, the total fat content in the area of interest - TFA decreased by 1.6 times, VFA and SFA - 2.1 and 1.6 times, respectively; VFA/SFA value was 0.22.3. The decrease in VFA was more intense, in compare to SFA, which led to a decrease in CRP by 3.5 times in 12 months after LSG.4. Hypertriglyceridemia is normalized in the first month after LSG, hypercholesterolemia after 6 months, the HDL level is restored to 24 months after LSG.5. A significant decrease in the AI and a decrease in the TG/HDL ratio indicates a decrease in cardiovascular risk as a result of LSG.
References
| [1] | Heymsfield Steven B, Thomas A. Wadden, Ph.D. Mechanisms, Pathophysiology, and Management of Obesity // N Engl J Med 2017; 376:254-66. DOI: 10.1056/NEJMra1514009. |
| [2] | Nazirov F.G., Khaybullina Z.R., Khashimov ShKh, Makhmudov U.M. Laparoscopic Sleeve Gasterectomy reduces inflammation and cardiometabolic risk in obese patients // Cardiovascular pharmacology: open access 5:200. doi: 10.4172/2329-6607.1000200., 2016. |
| [3] | Welbourn R., Marianne Hollyman1 & Robin Kinsman2 & John Dixon3 & Ronald Liem4 & Johan Ottosson5 &Almino Ramos6 & Villy Våge7 & Salman Al-Sabah8 & Wendy Brown9 & Ricardo Cohen10 & Peter Walton2 &Jacques Himpens Bariatric Surgery Worldwide: Baseline Demographic Description and One-Year Outcomes from the Fourth IFSO Global Registry Report 2018// Obesity Surgery https://doi.org/10.1007/s11695-018-3593-1. |
| [4] | Buchwald H., D. M. Oien. Metabolic/bariatric surgery worldwide 2011 // Obes. Surg. – 2013. |
| [5] | Reges O, Greenland P. Dicker D. Association of bariatric surgery using laparoscopicbanding, Roux-en-Y gastric bypass, or laparoscopic sleeve gastrectomy v usual careobesity management with all-cause mortality //JAMA 2018; (January). doi:10.1001/jama. 2017.20513. |
| [6] | Litvinova L.S., Vasilenko M.A., Zatolokin P.A. Adipocytokines production at patients with morbid obesity at various ways of treatment. Sakharnyi diabet [Diabetes]. 2014, no. 3, pp. 51-59. doi:10.14341/DM2014351-59. (in Russian). |
| [7] | Stegenga H, Haines A, Jones K, Wilding J. Guideline Development Group. Identification, assessment, and management of overweight and obesity: summary of updated NICE guidance.// BMJ 2014; 349: g6608. doi:10.1136/bmj.g6608 pmid:25430558. |
| [8] | Nazirov F.G., Khaybullina ZR, Sharapov N.U. Atherosclerosis and Metabolic Syndrome-significance of Inflammation, Urgency of Weight Loss and Extracorporeal Removal of Proinflammatory and Proatherogenic Substances // Cardiovasc Pharm Open Access 2017, 6:6. DOI: 10.4172/2329-6607.1000227. |
| [9] | Hadaegh F., Khalili D., Ghasemi A. et al. Triglyceride/HDL-cholesterol ratio is an independent predictor for coronary heart disease in a population of Iranian men // Nutr. Metab. Cardiovasc Dis. – 2009; №19(6): 401–408. doi: 10.1016/j.numecd.2008.09.003. |
| [10] | Salazar M.R., Carbajal H.A., Espeche W.G. et al. Identification of cardiometabolic risk: visceral adiposity index versus triglyceride/HDL cholesterol ratio // Am. J. Med. – 2014. – № 127 (2). – Р. 152–157. doi: 10.1016/j.amjmed.2013.10.012. |
| [11] | Tsiotra P.C., Boutati E., Dimitriadis G. et al. High insulin and leptin increase resistin and inflammatory cytokine production from human mononuclear cells.//Biomed Res Int. 2013, no. 20, pp. 487081. doi: 10.1155/2013/487081. |
| [12] | Heilbronn L.K., Campbell L.V. Adipose tissue macrophages, low grade inflammation and insulin resistance in human obesity//Curr. Pharm. Des. – 2008; № 14(12): Р.1225–1230. |
| [13] | Lemieux I, Poirier P, Bergeron J, Alméras N, Lamarche B, Cantin B, Dagenais GR, Després JP. Hypertriglyceridemic waist: a useful screening phenotype in preventive cardiology? // Can J Cardiol. 2007 Oct; 23 Suppl B:23B-31B. |
| [14] | Li Z, Deng ML, Tseng CH, Heber D. Hypertriglyceridemia is a practical biomarker of metabolic syndrome in individuals with abdominal obesity // Metab Syndr Relat Disord. 2013 Apr;11(2):87-91. doi: 10.1089/met.2012.0090. |
| [15] | Esser N., Legrand-Poels S., Piette J. et al. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes // Diabetes Res. Clin. Pract. – 2014. – № 105(2). – Р.141–150. doi: 10.1016/j.diabres.2014.04.006. |




 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML


