-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2019; 9(1): 409-416
doi:10.5923/j.ajmms.20190911.01

Immune System of Normal Endometry and Its Effect on the Development of Endometrial Cancer
S. V. Kamishov, M. N. Tillyashaykhov
Republican Specialized Scientific and Practical Medical Center of Oncology and Radiology of the Ministry of Health of the Republic of Uzbekistan, Tashkent, Uzbekistan
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The contribution of the immune system to the formation of tumors has not been studied for a long time, although it was already described for the first time several decades ago. However, more and more data have been accumulating for the past decade on the role that the immune system plays in the development and progression of a tumor and its possible role in the patient's prognosis. In addition, interest is growing for preclinical and clinical research concerning the use of the immune system in the treatment of cancer.Immunotherapy for gynecological cancers in general, and especially for endometrial cancer is still in its early days. Only a small number of studies, with varying success rates, have been published. We currently provide a concise overview of the available literature on the role of the immune system in the normal endometrium and in endometrial cancer as well as the possible implication for future immunotherapeutic studies.
Keywords: Immune system, Endometrium, Endometrial cancer
Cite this paper: S. V. Kamishov, M. N. Tillyashaykhov, Immune System of Normal Endometry and Its Effect on the Development of Endometrial Cancer, American Journal of Medicine and Medical Sciences, Vol. 9 No. 1, 2019, pp. 409-416. doi: 10.5923/j.ajmms.20190911.01.
Article Outline
1. Introduction
- Many of the risk factors associated with the etiology of endometrial cancer have been repeatedly described in the world literature. Obesity and lack of physical activity are two important risk factors for the development of uterine tumors, along with elevated blood pressure, high energy intake, high serum glucose and elevated exposure to estrogen [1]. Hormonal fluctuations during the menstrual cycle, as described, modulate the immune functions considered by Wira et al. [2]. Hormonal fluctuations and interactions with immune cells create a protective environment against the penetration of pathogenic microorganisms, creating a favorable environment for the implantation of embryos and the development of the fetus. Obesity which is associated with an increased risk of endometrial cancer, is considered a chronic inflammatory condition that causes an increased release of pro-inflammatory cytokines, such as IL-6 and CRP [3].In addition to the effects of the described risk factors on the immune system, the vast majority of endometrial cancers are diagnosed in postmenopausal women and often in elderly patients. Age has an important influence on the immune system, the so-called immune-senescence, which parallels hormonal changes occurring with increasing age [4]. Aging causes a general decline in immune functions and leads to a latent pro-inflammatory state.These data taken together indicate that risk factors associated with the onset of endometrial cancer have an important effect on the immune system. In the current review, we give an overview of the role that the immune system plays in a normal non-pregnant uterus, and how changes in the immune system can play a role in the development of uterine tumors and possible clinical outcomes. This knowledge is important for the further successful development of immunotherapeutic strategies for uterine cancer.
2. The Uterine Immune System under Physiological Conditions and in Cancer
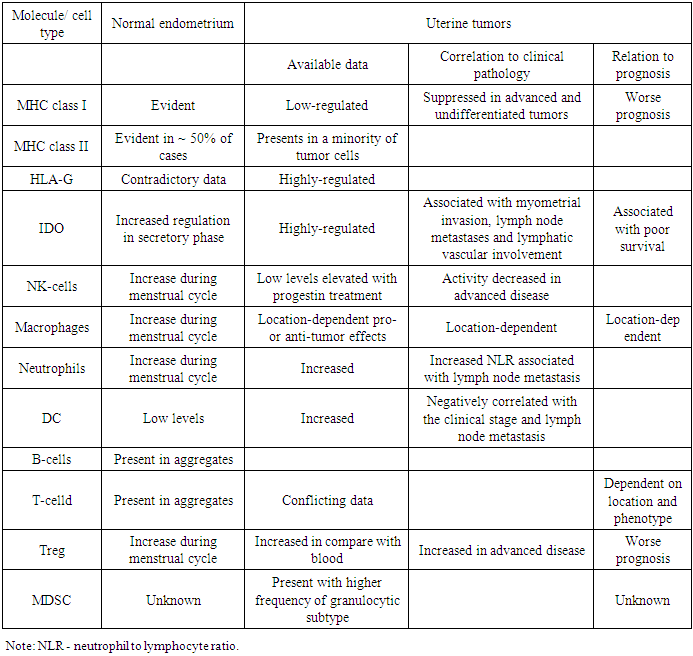
- The immune system in the normal uterus serves a dual purpose. On the one hand, it plays a role in protecting against pathogens, and on the other hand, it is able to adapt to the immunosuppressive state in order to create a feto-maternal tolerance to a semi-allogeneic fetus. These individual functions include the complex interaction of the hormonal fluctuations of the menstrual cycle and the immune system. Normal endometrium is naturally under strict hormonal control. He is under constant control of changes in estradiol and progesterone during the menstrual cycle. Both the innate and adaptive arm of the immune system are influenced by these hormonal changes. Several risk factors have been described for endometrial cancer, which may be linked with increased inflammation of the endometrial tissue, reviewed by Modugno et al. [5].It has been shown that increased exposure of estrogen is associated with the development of endometrial cancer due to the mitogenic effect of estrogen [6-8]. Therefore, estrogen-related carcinogenesis may be associated with inflammatory events. Chronic inflammation has been associated with cancer [9]. Several pathways of inflammation are involved in carcinogenesis. Many of these pathways are initialized, among other things, by activation of STAT 3 or NF-κB [10].Immune functions of normal and malignant endometrial cellsThe endometrial epithelium serves as the primary line of defense against viruses and other pathogens entering the uterus. The epithelial cells form an integral part of the mucosal immune system. Along with the formation of a physical barrier, epithelial cells perform several direct functions associated with the immune system, one of which is the secretion of defensins [11]. Defensins form a part of the innate immune system, considering their immediate antimicrobial function and their ability to activate the adaptive immune system. For example, it has been shown that defensins attract T-cells and immature dendritic cells (DC) in response to binding to the C-C chemokine receptor of type 6 (CCR6) [12]. Other secreted molecules include macrophage inflammatory protein (MIP) 3α, also a ligand for CCR6, and secretory leukocyte protease inhibitor (SLPI) [13-14]. Contradictory, uterine epithelial cells secrete unidentified, soluble immune mediators that confer a tolerogenic phenotype to DC [15].Obesity and diabetes have also been shown to be associated with increased release of pro-inflammatory molecules, such as IL-6, TNF-α, CRP, leptin and macrophage migration inhibitory factor [3, 16]. Two studies evaluating serum levels of IL-6, TNF-α and CRP and the risk of developing endometrial cancer showed that increased levels of CRP are associated with the risk of endometrial cancer [17-18]. Wang et al. found this correlation after correcting of BMI and age [18]. Friedenreich et al., in addition, found that the risk of CRP and endometrial cancer was associated with high BMI and serum IL-6, and the risk of endometrial cancer was associated with low BMI [17]. Indolamine 2,3-dioxygenase (IDO) which is responsible for suppressing T-cells by depriving T-cells of the essential nutrient tryptophan, is activated in the secretory and proliferative endometrium. The presence of the enzyme can fulfill a dual protective role: it acts as an antibacterial agent, while on the other hand it causes T-cell inhibition. The latter will create an immunosuppressive state to allow embryonal implantation [19]. IDO is also expressed by endometrial carcinoma cells [20–22], and it has been proven to be associated with myometrial invasion, lymph node metastases, and lymphatic vascular involvement [21]. In addition, the high expression of IDO correlated with a decrease in the involvement of CD8 + TIL and NK-cells and was associated with poor survival [20]. Thus, both in normal and malignant endometrium, IDO’s primary function seems to be induction of immunosuppression in order to allow embryonal implantation or tumor growth. Endometrial epithelial cells are also powerful antigen-presenting cells. Ferguson et al. found expression of MHC class I in endometrial glands as well as stromal cells and endothelial cells. MHC class II, on the contrary, was found to be expressed in the endometrial glands in approximately 50% of normal endometrium samples [23]. Fahey et al. have shown that cultured epithelial cells express CD40 and CD1d and that epithelial cells as well as stromal endometrial cells can elicit tetanus toxoid specific T-cell responses [24-25]. In endometrial tumors classic MHC of class I was suppressed in 48.5% of 520 tumors, which is associated with a worse prognosis of the disease [26]. In addition, the non-classical MHC class I molecule, HLA-G, was increased in 39.8% of samples, corroborating results of Barrier et al., who found expression of HLA-G in 55% of samples [27]. Despite the need for further study, the increased regulation of HLA-G molecules in endometrial tumors may be a protective mechanism to avoid NK-lysis of cells with reduced regulation of MHC class I molecules, as shown in other tumors [28-29]. MHC class II was found to be present in only a small portion of malignant endometrial cells. The low presence of both classical MHC I and II molecules and the activation of non-classical HLA-G indicate a weak antigen-presenting ability of endometrial tumor cells [30-31]. Cells in the underlying stroma, however, do show MHC II positivity [30]. Taken together, normal endometrial cells can present antigens in the context of MHC molecules, probably as a defense mechanism against pathogenic microorganisms. Endometrial tumor cells, however, inhibit the expression of MHC molecules to provide an immune output. Several members of the B7-H family have been described lately. We recently described the presence of these molecules both in normal endometrium and uterine tumors [22]. The expression of PD-L1 (B7-H1) and B7-H4 was detected in the vast majority of normal endometrium, while PD-L2 (B7-DK) was present in about half of the normal endometrium, albeit at low levels. All of these molecules were also present in endometrial cancer [22]. Comparing the expression levels of all molecules, no up-regulation in endometrial tumors was found. In spite of the fact that the studied population was rather small for a thorough analysis, there was a tendency to a decrease in survival in PD-L1 + tumors [22]. The results on B7-H4 are contradictory to a previously published study in which B7-H4 was reported to be significantly up-regulated in endometrial tumors [32,33]. The expression pattern of this molecule was mainly cytoplasmic in combination with strong membrane staining and, as it has been shown, negatively correlates with the number of TIL, both the T-cell population as a whole (CD3 +) and the individual CTL population (CD8 +) [32]. For the latter, this correlation was also found for B7-H3 [34]. These data indicate that, since for most of these mediators no up-regulation was found in endometrial tumors, these molecules may also exert their immunosuppressive functions in both the normal and cancerous situation, as described above for IDO. Table 1 gives a concise overview of the immunological players in endometrium and endometrial tumors and their implication in tumor biology.
|
3. Conclusions
- The data currently presented clearly show that the immune system is present and active in both normal endometrium and endometrial tumors. In the normal endometrium, the immune system plays a central role in protecting against pathogens and in providing tolerance to the feto-maternal line. Like this dual role in the healthy situation, it also has both a pro- and anti-tumorigenic function. In our opinion, the interplay between positive and negative players and mechanisms in tumor development and progression provides possible intervention options in the treatment of endometrial cancer, which deserves further attention in future research.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML