| [1] | Berkovic S.F., Knowiton R.C., Leroy R.F. et al. 2007, (Levetiracetam N01057 Stady Group). Placebo-controlled study of levetiracetam in idiopathic generalized epilepsy. Neurology., 69(18), 1751-1760. |
| [2] | Dmitrenko D.V., Shnayder N.A. 2014, Teratogenesis of antiepileptic drugs: review and clinical cases. Epilepsy and paroxysmal conditions., 6(2),61-70. |
| [3] | Voronkova K.V., Pylaeva O.A., Kosyakova E.S., Mazalskaya O.V., Golosnaya G.S., Provatorova M.A., Koroleva N. Yu., Akhmedov T.M., Ananyeva T V., Petrukhin A. S. 2010. Modern principles of treatment of epilepsy. Journal of Neurology and Psychiatry. Ss Korsakov., 110 (6), 24-36. |
| [4] | Voronkova K.V., Petrukhin A.S., Pylaeva O.A., Kholin A.S. 2008, Rational antiepileptic pharmacology. A guide for doctors. M: Bean Press, 192. |
| [5] | Dmitrenko D.V., Schneider N.A. 2014, Teratogenesis of antiepileptic drugs: a review of the literature and our own observations. Epilepsy and paroxysmal states., 6(2), 61-70. |
| [6] | Olga Tselousova, Olga Kochetova, Akhmadishina Leysan Zinurovna, Korytina Gulnaz Faritovna, Viktorova Tatyana Viktorovna 2009, Polymorphic variants of cytochrome P450 genes (CYP1A1, CYP2E1, CYP2D6) in the development of a predisposition to occupational toxicity. Acta Biomedica Scientifica.., 1, 136-140. |
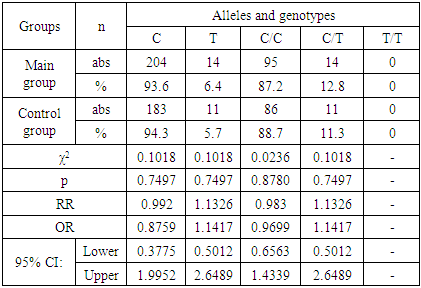
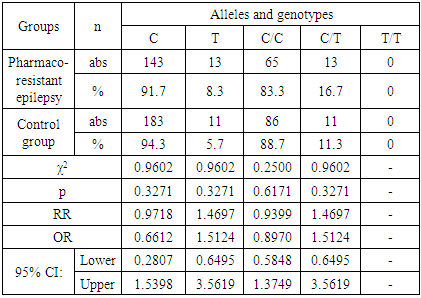
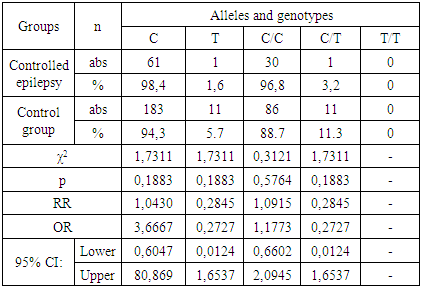
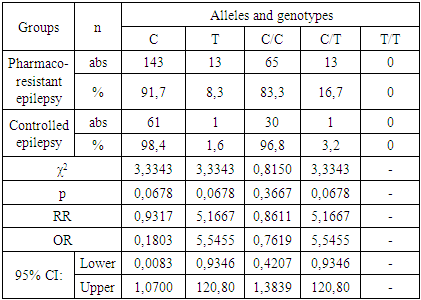
| [7] | Schneider N. A., Dmitrenko D.V., Govorina Yu.B., Muraveva A.V., Kotlovsky Yu.V., Bochanova E.N., Fateeva E.A., Dedyuk N.A., Mustafayeva A. 2015, The effect of CYP2C9 gene polymorphisms on the level of valproic acid in the blood of women of reproductive age with epilepsy. Pharmacogenetics and pharmacogenomics., 24-28. |
| [8] | Schneider N.A., Pilyugina M.S., Dmitrenko D.V. 2011, Stratification of patients with epilepsy by risk groups for the development of undesirable medicinal phenomena in patients receiving valproic acid preparations., 7 (62), 50-63. |
| [9] | Atsuko Odani, Yukiya Hashimoto, Yuko Otsuki Yuichi Uwai Haruo Hattori , Kenshi Furusho, Ken‐ich Inui 1997, Genetic polymorphism of the CYP2C subfamily and its effect on the pharmacokinetics of phenytoin in Japanese patients with epilepsy. Pharmacokinetics and Drug Disposition., 62(3), 287-292. |
| [10] | Brian B. Spear, 2008, Pharmacogenetics and Antiepileptic Drugs., Epilepsia, 42(s5), 31-34. |
| [11] | Simon C., Stieger B., Kullak‐Ublick G. A., Fried M., Mueller S., Fritschy J.‐M., Wieser H.G. and Pauli‐Magnus C., 2006, Intestinal expression of cytochrome P450 enzymes and ABC transporters and carbamazepine and phenytoin disposition., Acta Neurologica Scandinavica, 115, 4, 232-242. |
| [12] | Cassandra E. I., Szoeke M. B., MarkNewtonc Julie M. Wood, David Goldstein, Samuel F. Berkovic, Terrence J., O. Brien, Les J. Sheffieldaf 2006, Update on pharmacogenetics in epilepsy: a brief review., 5(2), 189-196. |
| [13] | Fricke-Galindo I., Jung-Cook H., Lerena A. L., López-López M. 2018, Pharmacogenetics of adverse reactions to antiepileptic drugs. Farmacogenética de reacciones adversas a fármacos antiepilépticos., Neurología, 33(3), 165-176. |
| [14] | HariOm Singh, Sonam Lata, Vijay Nema, Dharmesh Samani, Manisha Ghate and Raman R. Gangakhedkar, 2017 CYP1A1m1 and CYP2C9*2 and *3 polymorphism and risk to develop ARV‐associated hepatotoxicity and its severity., APMIS, 125, 6, (523-535). |
| [15] | Hung, Chin-Chuan, Lin, Chun-Jung, Chen, Chih-Chuan, Chang, Chee-Jen, Liou, Horng-Huei 2004, Dosage Recommendation of Phenytoin for Patients with Epilepsy with Different CYP2C9/CYP2C19 Polymorphisms., Therapeutic Drug Monitoring: October– 26(5), 534-540. |
| [16] | Jung‐Woo Bae, Chang‐Ik Choi, Choon‐Gon Jang and Seok‐Yong Lee, 2011, Effects of CYP2C9*1/*13 on the pharmacokinetics and pharmacodynamics of meloxicam, British Journal of Clinical Pharmacology, 71, 4, 550-555. |
| [17] | Karthik Venkatakrishnan, Lisa L. Moltke and David J. Greenblatt, 2013, Human Drug Metabolism and the Cytochromes P450: Application and Relevance of In Vitro Models., The Journal of Clinical Pharmacology, 41(11), 1149-1179. |
| [18] | Schwarz U. I. 2003, Clinical relevance of genetic polymorphisms in the human CYP2C9 gene, European Journal of Clinical Investigation., 33, 23-30. |
| [19] | van der Weide, Jana; Steijns, Linda S. W.b; van Weelden, Marga J. M.c; de Haan, KeimpecThe effect of genetic polymorphism of cytochrome P450 CYP2C9 on phenytoin dose requirement. Pharmacogenetics: June 2001 - Volume 11 - Issue 4 - p 287-291. |
| [20] | Wiparat Manuyakorn, Khanitha Siripool, Wasu Kamchaisatian, Samart Pakakasama, Anannit Visudtibhan, Soamarat Vilaiyuk, Thidarat Rujirawat, Suwat Benjaponpitak, 2013, Phenobarbital‐induced severe cutaneous adverse drug reactions are associated with CYP2C19*2 in Thai children, Pediatric Allergy and Immunology, 24(3), 299-303. |
| [21] | Yingjie Guo, Cheng Hu, Xiaojing He, Feng Qiu, Limei Zhao 2012, Effects of UGT1A6, UGT2B7, and CYP2C9 genotypes on plasma concentrations of valproic acid in Chinese children with epilepsy., J.-STAGE, Drug Metabolism and Pharmacoki ...DMPK., 27(5), 536-542. |


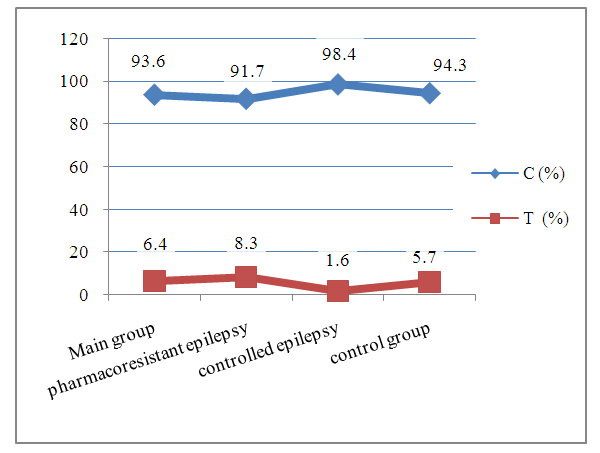
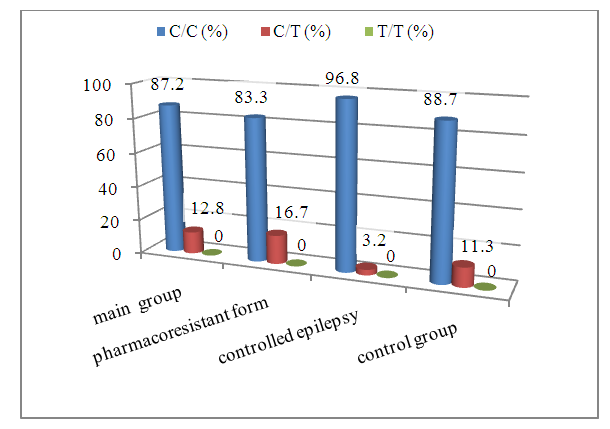
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML