-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2019; 9(0): 365-371
doi:10.5923/j.ajmms.20190910.03

Estimation of Results and Microsurgical Aspects of Cisterotomy at Severe Craniocerebral Injury
K. E. Makhkamov, A. B. Salaev, M. K. Makhkamov
Republican Research Centre of Emergency Medicine, Tashkent, Uzbekistan
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The article provides a comparative evaluation of the surgical treatment results in patients with severe craniocerebral injury. The research is based on a retrospective analysis of 56 patients treated at the Republican Research Centre of Emergency Medicine. Patients were conditionally divided into two groups: the 1st - patients with traditional decompressive hemicraniectomy; the 2nd - a group of patients with decompressive hemicranectomy with augmented cisternotomy of the brain basal cisterns. Technique of surgical intervention has been described in detail. During the postoperative period, the size of the bypass and bridge tank was estimated using the control tomography of the brain. The estimation of patient’s condition was evaluated according to the Glasgow outcome scale.
Keywords: Traumatic brain injury, Decompressive craniotomy, Brain cisterns
Cite this paper: K. E. Makhkamov, A. B. Salaev, M. K. Makhkamov, Estimation of Results and Microsurgical Aspects of Cisterotomy at Severe Craniocerebral Injury, American Journal of Medicine and Medical Sciences, Vol. 9 No. 0, 2019, pp. 365-371. doi: 10.5923/j.ajmms.20190910.03.
Article Outline
1. Introduction
- Severe craniocerebral injury (SCCI) is one of the urgent problems of modern neurosurgery. According to WHO, there is an annual increase in craniocerebral injury by an average of 2% per year. SCCI is often called the "silent epidemic" because of this high prevalence. This phenomenon is considered by some sociologists as the reverse side of the scientific and technological revolution, payment for increased speeds and new technologies [1]. The prevalence of this phenomenon reaches 4–7.2 cases per 1000 population in various regions [2]. The introduction of decompressive hemicraniectomy (DH) and the improvement of intensive care with the control of intracranial pressure (ICP) has become the most significant step in the surgical treatment of severe craniocerebral injury [3]. There are no clear indications for DH in modern guidelines on the treatment of SCCI, but it is only stipulated that “under certain conditions DH can be performed as a treatment for cerebral edema (CE) and intracranial hypertension (ICH), refractory to conservative therapy” [4]. Such uncertainty is associated with developing complications in the postoperative period, with continued high mortality and unsatisfactory functional outcomes of treatment after DH. In the early postoperative period increasing cerebral edema promotes incarceration and the formation of strangulation with the development of secondary hemorrhagic and ischemic complications. After GH new or expanded hemorrhagic contusions of >or=5 cc were observed after hemicraniectomy in 58% of patients [5] and the contralateral-side hematoma that formed after the acute hematoma was an epidural hematoma in eight (66.7%), a subdural hematoma in two (16.7%), and an intracerebral hematoma in three (25.0%) cases [6]. Recent researches suggest that the formation of cerebral edema is associated with the entry of cerebrospinal fluid (CSF) into the brain parenchyma through the low resistant Virchow-Robin spaces (VRS). The authors observed bulk flow CSF movement along para-arterial pathways directly using in vivo two-photon imaging of intracisternally injected TR-d3 and FITC-d2000. Both of these agents moved rapidly along the wall of pial arteries to enter the VRS without mixing with CSF in the surrounding subarachnoid compartment. It indicates that the paravascular space around surface arteries and the Virchow-Robin space into which these penetrate comprise a physically and functionally distinct subcompartment through which CSF rapidly enters the brain parenchyma by bulk flow [7]. The use of the technique of the brain arachnoid cisterns dissection during edema allows to create a reverse outflow of liquor through VRS, decreasing ICH and reducing trauma of axonal stretching during brain bulging, and ultimately to lessen a mortality [8].However, the study and improvement of surgical techniques for injuries remained practically absent, while non-surgical methods of treatment and research in the field of conservative treatment with therapeutic options for neuroprotection of SCCI are quickly gaining popularity [9]. The improvement of optical systems, micro-tools and a detailed understanding of changes in liquor dynamics during the SCCI period show the need for research by improving surgical techniques.
2. Aim
- The aim of our study was a comparative estimation of surgical treatment results in patients with severe craniocerebral injury.
3. Materials and Methods
- Our study is based on a retrospective analysis of 56 patients treated in the Republican Research Center of Emergency Medicine with severe craniocerebral injury (SCCI). There were 48 males and 8 females at the age of 18-62 years (mean age was 47.3±5.3 years). The criterion of the patient selection for this study was a violation of consciousness by the Glasgow Coma Scale (GCS) less than 8 points. Anamnesis, neurological examination according to generally accepted protocols and physical examinations were conducted. Multislice computed tomography (MSCT) was performed for all patients to assess the nature of contusion foci according to the “Janett” classification, intracranial hemorrhage and its volume, the severity of the dislocation syndrome, the state of the ventricular system and basal cisterns of the brain. In order to study the adequacy of cisternostomy in the surgical treatment of SCCI patients were divided into two groups: Group 1 – 39 patients with traditional DH; Group 2 – 17 patients with DH supplemented by cisternostomy of the brain basal cisterns. Evaluation of treatment results was carried out using the Glasgow Outcome Scale (GOS). Statistical processing of the results was evaluated using the “Statistica 6.0” program. The average values of the sizes of the bypass and bridge tanks in the preoperative and postoperative periods were calculated in each group. The reliability of the results was assessed by Student's t-test (f = 49, α = 0.05).
4. Surgical Technique
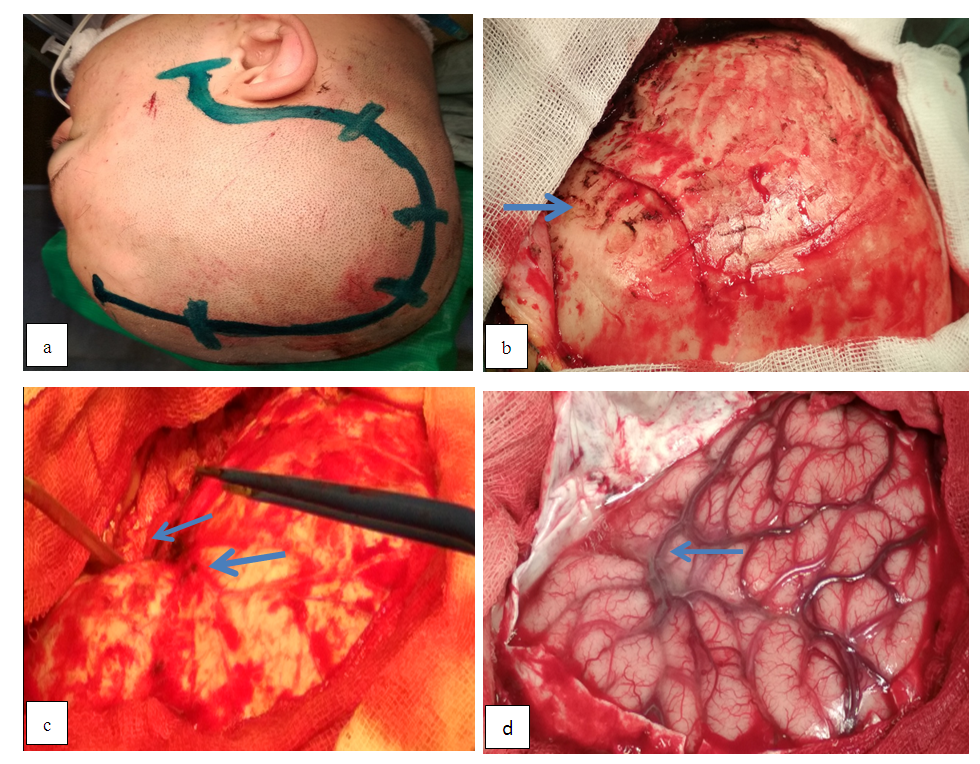
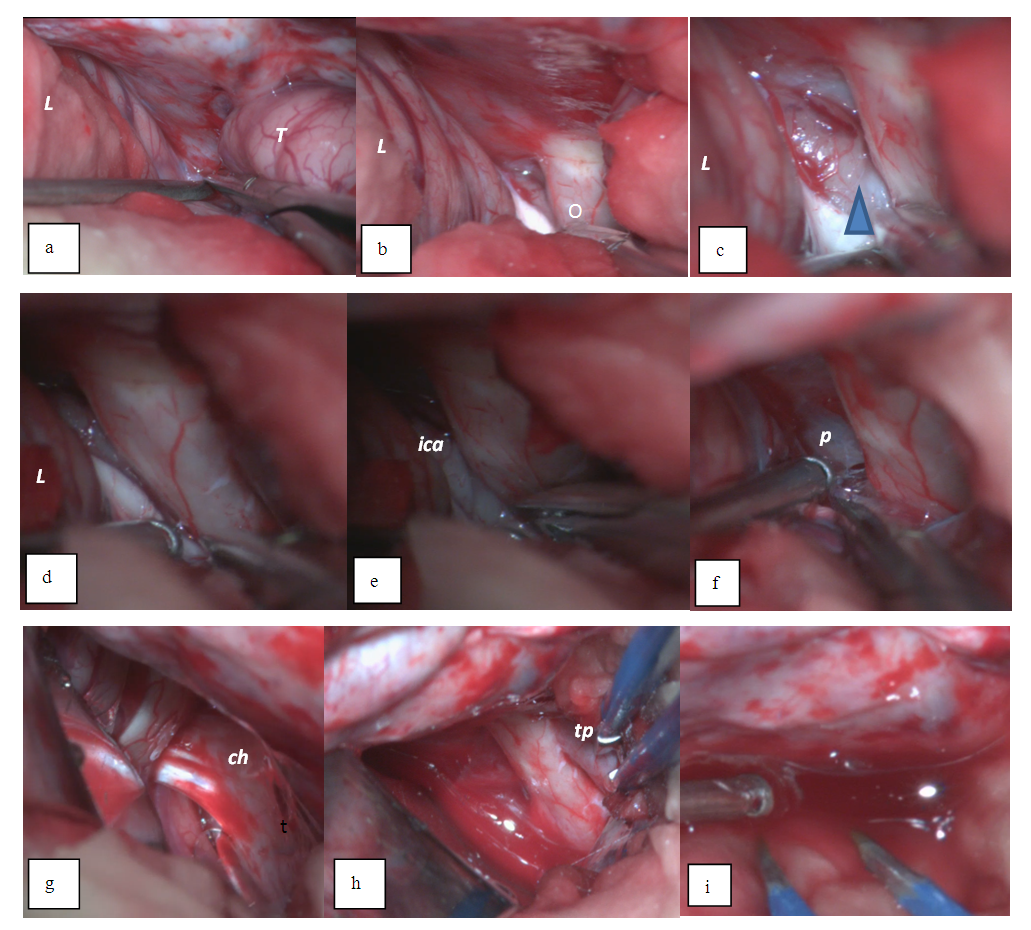
- Head laying. The patient's head is fixed on the Mayfield bracket which consists of three-point fixation. The head is positioned above the heart level by 30° and rotated laterally to the contra-lateral side by 45° to achieve a wide decompressive window and an optimal viewing angle of the skull base to perform a cisternostomy.Incision of skin and craniotomy. Crescent-shaped marking of the incision line with the base of the orbital-zygomatic region is performed in the fronto-parietal-temporal zone and in the area of a vasoconstrictor introduction along the incision line into the soft tissues, then an incision is made in the skin and underlying soft tissues to the bone and temporal muscle.It should be noted that the incision line does not descend to the level of the zygomatic arch which makes it possible to avoid injury of the superficial temporal artery. The temporal muscle is dissected along the wound edge using an electrocautery and a single skin-musculoaponeurotic flap is formed and retracted using special retractors after separation towards the base and the separation takes place until the outer edge of the orbit, the zygomatic process of the frontal bone and the pterional area. After the skeletonization of the frontal, parietal and temporal bones, a milling hole is placed over the projection of the parietal mound through the which hemicranectomy is performed.Stitching the skull base and opening the dura mater. The lateral part of the anterior cranial fossa (orbital roof) and the crest of the main bone are grinded using a high-speed drill. First, a 5 mm cutting tip under the irrigation solution is used to grind the bone, and then a 5 mm diamond attachment is used to grind bone and bone hemostasis. Grinding is performed without irrigation of the solution by hot polishing with the purpose of bone hemostasis. Stitching of the orbit roof is performed up to the achieving of a single angle of view with the anterior oblique process. The comb of the main bone is also grinded to achieve a single plane with the lateral part of the anterior cranial fossa and the initial parts of the anterior inclined process, thereby achieving decompression of the Sylvian gap (SG) projection and its anatomical structures. Epidural hematomas are removed with a search and bipolar coagulation of the bleeding source. The dura mater (DM) is opened horseshoe-shaped to the base of the skull and with the help of interrupted sutures is tightened around the perimeter for reducing the residual space between the bone. One and / or two sutures are applied to the DM base which additionally opens up the space and at the same time reduces the traction of the medulla. After opening the DM, the intracranial hematoma of a particular site is removed (Figure 1).
5. Results
- The results of our study showed that in both groups the average hematoma volume was 74.4 ± 24.7 cm3. In the first group of patients subdural hematoma combined with contusion foci of type 2-3 was diagnosed in 16 (47.6%) patients, epidural hematoma combined with contusion foci of type 2-3 – in 13 (38.2%) cases and multiple hematomas combined with contusion foci of type 2-3 were diagnosed in 10 (29.4%) patients. In the second group 13 (76.5%) patients had subdural hematomas combined with contusion foci of type 2-3. Four (23.5%) patients had multiple hematomas, i.e. epi- and subdural hematomas combined with contusion foci of type 2-3. The estimation of the brain bypass and bridge cisterns on the control MSCT 1, 3 days after the operation showed that in the second group of patients a regression of bypass cistern compression was observed on average by 5.4 ± 0.8 mm compared with patients of the first group, where the regress made up 3.5 ± 0.4mm. When evaluating the condition of the bridge cistern we noted that also in the second group of patients, compared with the first one, there was a significant regression of this cistern compression by an average of 2.9 ± 0.3 mm versus 1.7 ± 0.4 mm. MSCT scans data at the level of basal cisterns before and after surgery due to CCI are shown in Figures 3-7.
 | Figure 3. MSCT images of the patient before the surgery - gross dislocation syndrome, brain compression with acute subdural hematoma, compression of the bypass and bridge cisterns are noted |
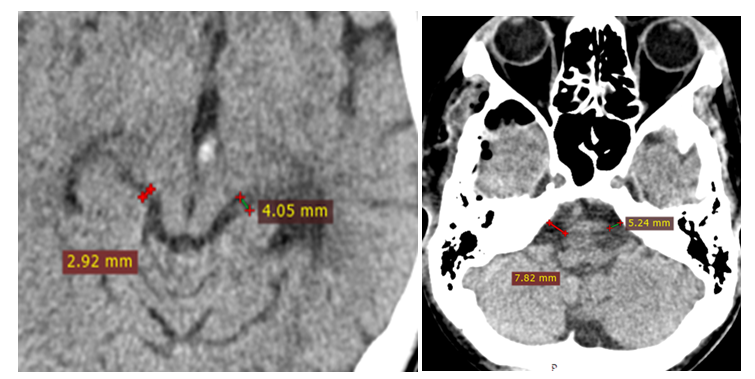 | Figure 4. MSCT images of the same patient 2 days after the surgery of decompressive trepanation with augmented cisternotomy, straightening of the bypass and bridge cisterns |
 | Figure 5. MSCT images of the patient before the surgery, evident dislocation syndrome, bypass cistern is not visualized |
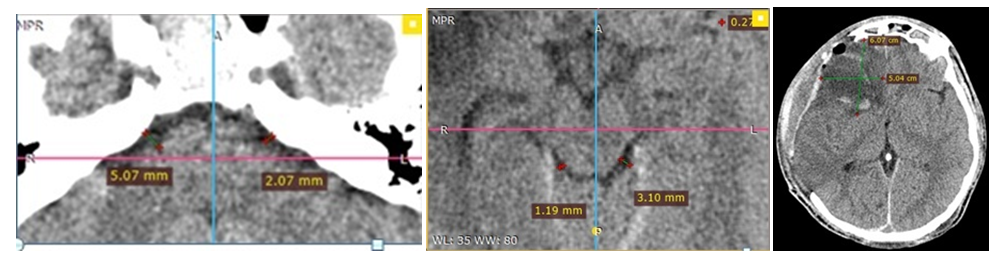 | Figure 6. MSCT images of the same patient 2 days after the surgery of decompressive craniotomy without a cisternotomy, compression of the ventricular system and basal cisterns is maintained |
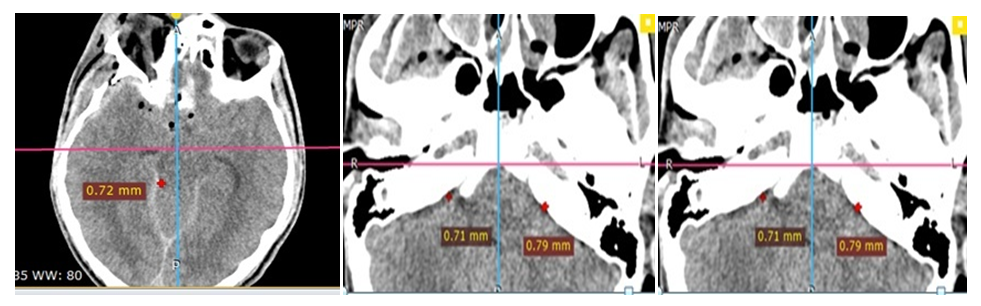
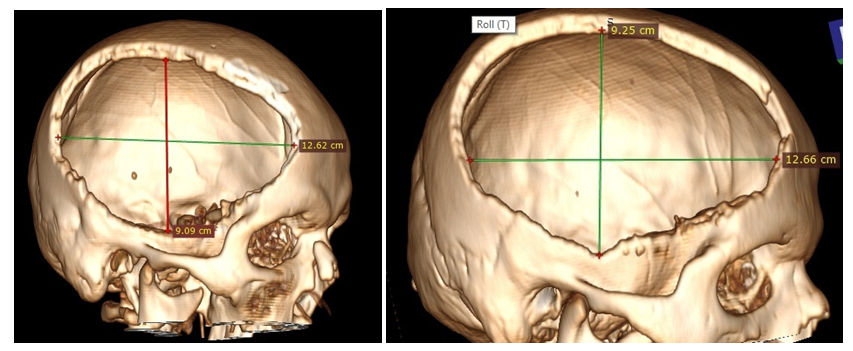 | Figure 7. MSCT 3D skull defect reconstruction - no difference in the size of the bone window |
6. Discussion
- According to a number of authors, the deformation and compression of cisterns of the brain base during computed tomography are pathognomonic symptoms of brain stem compression in victims with SCCI [10,11]. In this connection, surgical treatment (decompressive trepanation) is carried out immediately, on an emergency basis, until the onset of secondary circulatory disorders in the brainstem [12]. The search for new ways in the surgical treatment of patients with SCCI is often caused by poor outcomes of surgical treatment, such as the progression of traumatic foci in 58% of patients [5], hydrocephalus in 26 patients [13], secondary ischemic brain injury in 56 patients, an increase in ICH and progression of brain edema [14]. Currently, the introduction of microsurgical techniques in emergency neurosurgical care is often difficult due to the lack of recommendations and standards for the use of techniques and technical difficulties during the surgery: severe bleeding and the development of abrupt brain swelling with a simultaneous drop in blood pressure which often leads to speeding surgical intervention up. The use of microsurgical approaches in the treatment of patients with temporal- herniation at craniocerebral injury is found in the literature in the form of selective microsurgical resection of the strangulated hook of the parahippocampal gyrus in combination with open tentoriotomy for additional internal decompression (Chibbaro et al., 2008). The results of surgical treatment of a temporal-tentorial herniation in 80 patients with severe isolated craniocerebral injury and suppression of wakefulness to coma (8 points or less by GCS) are described. Favorable treatment outcomes were obtained in 75% of patients, outcomes with severe neurological deficit - in 10%, mortality was 15% [15]. The technique of wide, temporal-decompressive craniotomy with a radical removal of brain injury foci with subsequent selective microsurgical resection of the anterior sections of the middle and lower temporal convolutions and the hook of the hippocampal gyrus in 10 cases has been described. An excellent treatment outcome was recorded in one patient from 10 (10%). Moderate disability was observed in 3 (30%) patients, severe disability - in 2 (20%). Mortality was 4 (40%) [16]. Despite the routine use of methods of intravital neuro-imaging, the continuous improvement of microsurgical instruments and equipment of operation rooms, the temporal lobe resection as a method of internal decompression of the brain is not widely used in emergency neurosurgery [17]. The use of cisternostomy of the brain basal cisterns as a microsurgical method of surgical treatment for severe traumatic brain injury with the aim of additional internal decompression of the brain corresponds to new approaches of liquor circulation concept, the role of cerebrospinal fluid in the pathogenesis of Virchow-Robin parivascular spaces [8]. Surgical treatment is no less complex requiring the use of modern optical technology and the experience of a surgeon in emergency medicine. The literature describes 39 cases of drainage of basal cisterns in the treatment of craniocerebral injury followed by early rehabilitation treatment and a good outcome by Glasgow scale (1.9) [18]. The experience of using cisternostomy with DH in children and adults with SCCI is described in 218 cases. The average value by the Glasgow scale after 6 weeks was 3.7 and the mortality rate was 26.4% [19]. In our study we presented the first experience of using DH with cisternostomy in victims with SCCI. The obtained data of clinical and instrumental examination of patients in the postoperative period confirm the high efficiency of this method of the brain internal decompression. Thus, in patients who underwent DH with cisternostomy, a more rapid elimination of basal cistern compression according to MSCT was observed in the dynamics. It led to a faster recovery of wakefulness after surgery than in DH patients with no deaths and a vegetative state.
7. Conclusions
- At present, approaches to the use of typical DH at SCCI require revision with the introduction of microsurgical interventions into widespread practice of the emergency medicine system. Good results obtained by us in applying cisternostomy of basal cisterns testify to this. However, many questions remain to be answered: selection of patients, the number of opened cisterns, determination of the intraoperative sufficiency level and the ultimate goal of opening the cisterns, the effect on long-term results and outcomes in patients that we hope to explore and present later. When studying all the questions, we hope to find the optimal path to primary and complete neurosurgical care excluding repeated interventions in patients with SCCI. Our study showed that performing decompressive hemicraniectomy with cisternostomy for severe craniocerebral injury can not only reduce adverse outcomes, but also reduce the number of gross disability.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML