-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2019; 9(0): 361-364
doi:10.5923/j.ajmms.20190910.02

Study of the Gene-Geographical Profile of the Population among Donors in Tashkent on the Antigenic Composition of Erythrocytes
Gulnoz Khabibjonovna Kayumova
Department of Extracorporeal Blood Purification, Scientific Research Institute of Hematology and Blood Transfusion of the Ministry of Health of the Republic of Uzbekistan, Tashkent, Uzbekistan
Correspondence to: Gulnoz Khabibjonovna Kayumova, Department of Extracorporeal Blood Purification, Scientific Research Institute of Hematology and Blood Transfusion of the Ministry of Health of the Republic of Uzbekistan, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The purpose of the study was to study the genogeographic profile of the population on the antigenic composition of erythrocytes. In the blood samples of donors in the city of Tashkent, diagnostics of allogeneic immunization of the population and the determination of anti-erythrocyte antibodies were carried out. It has been established that the distribution by clinical important antigens of the Rh-Hr system among the donors of the city of Tashkent, which corresponded to similar indicators among Europeans. It is necessary to immunophenotype donors and recipients not only for antigens A, B and D, but also for antigens C, C, E, e.
Keywords: Genogeographic profile, Erythrocyte antigens, Anti-erythrocyte antibodies, Allogeneic immunization, "Rh-Hr" system
Cite this paper: Gulnoz Khabibjonovna Kayumova, Study of the Gene-Geographical Profile of the Population among Donors in Tashkent on the Antigenic Composition of Erythrocytes, American Journal of Medicine and Medical Sciences, Vol. 9 No. 0, 2019, pp. 361-364. doi: 10.5923/j.ajmms.20190910.02.
Article Outline
1. Introduction
- Allogeneic immunization resulting from the transfusion of erythrocytes carrying transfusion dangerous antigens on their surface are the main cause of posttransfusion disease (PTD) and hemolytic disease of the newborn (HDN) [17]. Erroneous conclusion about the presence or absence of antigens and antibodies in donors and recipients can lead to the development of post-transfusion disease (PTD) of hemolytic type [1-3,8,9,14,15]. Maternal antibodies, which easily cross the placental barrier and enter the fetal bloodstream, are of key importance in the development of hemolytic disease of the newborn (HDN). The degree of development of fetal erythrocyte hemolysis depends on the specificity of antibodies. Despite the achievements in perinatal diagnostics, in particular, in prevention and treatment, it is not possible to completely prevent morbidity and mortality of children from hemolytic disease. Immunohematological studies in the mother help to predict the possibility of development and severity of hemolytic disease, to optimize the hemotransfusion therapy of newborns, as well as to carry out the selection of blood components for women [2,3,8,18,19,28].Scientific researchers know about 38 group antigenic systems, including more than 500 erythrocyte antigens that can be identified using specific antisera. The combination of group antigens individually for each person [12]. The frequency of blood groups among representatives of different races and ethnic groups varies, which is believed to be a consequence of the genogeographic adaptation to one or another ecosystem in the process of evolution [6,7,10,21,28].In our republic, only 6 antigens are determined by two systems - ABO and Rhesus, and screening of immune antibodies is carried out in blood service institutions and health care facilities (MPI), but the absence of a "panel of standard erythrocytes" does not allow to determine the specificity of detected antibodies [25].According to the literature data (S.I. Donskov, T.V. Gaponova, 2013) such an approach to determining blood groups can ensure the safety of blood transfusion by no more than 80% [4,5,11]. Other antigens, such as E, e, C, K, K, CW, etc., which are not associated with the danger of transfusions, are not determined by the blood service. However, according to WHO recommendations (2005), blood transfusion should be carried out after determining at least 10 transfusion-dangerous antigens.
2. Main Body
2.1. The Purpose of Our Research
- Study of the genogeographic profile of the population on the antigenic composition of erythrocytes.
2.2. Material and Methods of Study
- This study involved 320 conditionally healthy donors surveyed on the basis of the Republican Center for Blood Transfusion and on the basis of the clinical diagnostic department of the Scientific-Research Institute of Hematology and Blood Transfusion of the MH RUz.The material for the study was whole blood, red blood cell mass, plasma, blood serum from donors in Tashkent. The following research methods were used: isoserological methods, using monoclonal reagents of different specificity, determination of anti-erythrocyte antibodies using antiglobulin serum and conglutination method, using 33% polyglucin solution.The method of diagnosing alloimmunization of the population was carried out using monoclonal reagents of different specificity and the determination of anti-erythrocyte antibodies using antiglobulin serum and conglutination using a 33% polyglucin solution. Transfusion erythrocyte erythrocyte antigens from the Rhesus, Kell and Kidd systems were determined using monoclonal antibodies, which are produced by in vitro hybridoma cell lines [16,20].Statistical data processing was performed using Microsoft Excel 2007.
2.3. Results of the Study
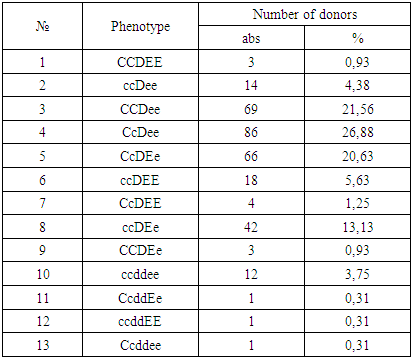
- A donor test for the presence of incomplete immune antibodies using a 33% polyglucin and in an indirect Coombs test showed the following results: of 7878 antibody donors were detected in 19 serum samples. The titer of incomplete forms of immune antibodies ranged from 1: 2 to 1: 128. Serum samples that yielded positive results in the determination of antibodies can contain both “pure” anti-D antibodies and simultaneously antibodies to several antigens: anti-C + D + E and others.To determine the specificity of antibodies, a special study of serum with a set of standard erythrocytes, including the rare ccDee, Ccddee, ccddEe, ccddee groups, including both containing and not containing Kell (K) and Duffy (Fy) antigens, is necessary. To determine the specificity of the antibody was carried out to develop a panel of standard erythrocytes. A total of 21 samples of erythrocyte O (I) blood group donors were selected and immunotyped (of which 20 are Rh-positive, 1 is Rh-negative, 1 is Kell-positive, 1 is CW-positive). Compiled electronic file of typed donors in alphabetical order. The results of the distribution of phenotypes of the "Rh-Hr" system among the donors of the city of Tashkent are given in the following table 1.
|
3. Conclusions
- Among the donors in Tashkent, the distribution of the clinically important antigens of the Rh-Hr system (except for antigen D) corresponds to similar indicators in Europeans.To prevent the development of post-transfusion disease, it is necessary to immunophenotype donors and recipients not only for antigens A, B and D, but also for antigens C, C, E, E.The obtained data will help the attending physicians to orient in the selection of compatible donor-recipient pairs, reduce the risk of post-transfusion disease (PTD), and blood service specialists to regulate the production of the necessary blood-transfusion components and improve the blood immunohematological safety.
ACKNOWLEDGEMENTS
- The author thanks the leadership of the Republican Center for Blood Transfusion of the MH RUz and personally to the Director Alonur Bakhtinurovich Saidov for assisting in the organization of the selection of the studied group of donors. The author also thanks the head of the Clinical Diagnostic Department of the Research Institute of Hematology and Blood Transfusion of the MH RUz Nigora Akhrarova for their assistance in organizing the implementation of laboratory tests.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML