-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2019; 9(0): 357-360
doi:10.5923/j.ajmms.20190910.01

Molecular – Genetic Diagnostics in the Study of Hysteromyoma Progression
L. M. Isanbayeva, D. A. Kadirova
Tashkent Institute of Post-Educational Doctors, Tashkent Institute of Biophysics and Biochemistry at the National, University of Uzbekistan, Uzbekistan
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In spite of significant progress in the study of various hysteromyoma, a number of key issues such as the identification of genetic factors and primary molecular defects leading to the development and evolution of myomatous uterine changes, early diagnostics still remain insufficiently studied. This article shows that there is a higher expression of Bcl-2 proto-oncogene in myomatous nodes, which is one of the main inhibitors of apoptosis. Increased expression of Bcl-2 in the cell leads to a change in the normal course of apoptosis and the life span of this cell is increased. The Bcl-2 gene being one of the regulators of the balance between cell proliferation and cell death is of great importance in tumor growth. High expression of this gene is associated with a low level of apoptosis, i.e. there is an inverse correlation between the p53 and Bcl-2 genes.
Keywords: Hysteromyoama, Proliferation, Apoptosis, Proto-oncogene
Cite this paper: L. M. Isanbayeva, D. A. Kadirova, Molecular – Genetic Diagnostics in the Study of Hysteromyoma Progression, American Journal of Medicine and Medical Sciences, Vol. 9 No. 0, 2019, pp. 357-360. doi: 10.5923/j.ajmms.20190910.01.
1. Introduction
- Hysteromyomas are benign proliferative diseases of the uterus. A significant increase in the frequency of the uterus benign proliferative diseases begins in the late reproductive age and there has been a significant "rejuvenation" of patients contingent for recent years [1-4]. The study of the etiology, clinical and pathogenetic variants of the hysteromyomas course, the pathogenetic factors of their development and progression are not well covered in the literature. The use of modern treatment methods does not always provide a high therapeutic effect, the problem of the disease recurrence remains actual. In this regard, the search of new diagnostics and treatment methods based on an in-depth study of the uterus proliferative diseases pathogenesis is being continued. More and more attention in this area has been recently paid to various molecular and cellular markers characterizing the fundamental biological properties of a tumor, namely, to proliferative capacity, activity of apoptosis, and neoangiogenesis in tumors. An important role in the origin and development of tumors of the p53 gene, which protects the body from mutant cells was noted [5]. The main function of the p53 protein is to protect the integrity of the cell genome. DNA damage serves as a signal for the p53 gene and for the start of apoptosis. By blocking the Bcl-2 gene, via p53, the mechanism of apoptosis is triggered, preventing infinite, uncontrolled cell division. The p53 protein functions as a tumor suppressor; accordingly, the p53 gene is an anti-oncogene. Mutations of this gene allow to maintain mitotic activity to the cells with damaged DNA. It is known that in regulating the balance of such physiological processes as proliferation, differentiation and programmed cell death belongs to the Bcl-2 gene. Mutations of the p53 gene allow cells with damaged DNA to maintain mitotic activity. Incompleteness of apoptosis explains the different sizes and different degrees of the nodes maturity, the benign nature of the tumor, non-invasive and slow tumor growth. Bcl-2 protein plays a key role in the regulation of apoptosis [6,7]. Myomatous nodes have a higher content of Bcl-2 proto-oncogene which is one of the main inhibitors of apoptosis [8]. Increased expression of Bcl-2 in the cell leads to a change in the normal course of apoptosis and increases the lifespan of this cell. Being one of the regulators of the balance between proliferation and cell death the Bcl-2 gene is of great importance in tumor growth. High expression of the p53 gene is associated with a low level of apoptosis, i.e. there is an inverse correlation between the p53 and Bcl-2 genes [10]. Based on this, analysis of the p53 gene and the Bcl-2 proto-oncogene expressions, the dependence of their expression on various factors during tumor formation are an important pathophysiological characteristics.The aim is to analyze the expression of p53 and Bcl-2 genes in women with different clinical course of hysteromyoma and their changes against the background of the conducted therapy.
2. Material and Methods
- Samples of 200 patients who were treated at the City Perinatal Center and the City Maternity Center No. 3 served as the material for molecular-genetic research. The main group was consisted of 180 people with a histologically verified diagnosis of hysteromyoma, 20 patients made up the control group. The blood of women with various hysteromyoma was used as the material. Blood in a volume of 1 ml was taken using a catheter from the ulnar vein into vacuum tubes with 0.5 m of EDTA.Excretion of nuclear DNA from blood leukocytesAll procedures were performed in sterile conditions. 0.5 ml of peripheral blood was added by 2 ml of sterile H2O, the samples were incubated for 2 hours at room temperature. Leukocyte cells were besieged at 3000 rpm, 40°C for 15 minutes. The supernatant was carefully removed, 100 μl of sterile distilled water was added to the yellow-red leukocyte sediment and stored at -20°С. 1 ml of lysis buffer (100 mM Tris-HCl, pH 7.6; 25 mM EDTA pH 8.0; 0.15 M NaCl; 1% SDS, 2 mM β-mercaptoethanol) was added to a suspension of leukocytes (1x106), lysis was performed over ice for 3 minutes. Then the samples were centrifuged at 3000 rpm, 40°C, 15 min. The supernatant was transferred to new tubes, an equal volume of phenol-chloroform mixture (1: 1) was added, incubation for 15–20 min with shaking. Samples were centrifuged for 10 minutes at 5000 rpm, 40°C. Chloroform (1: 2) was added to the supernatant, incubation with shaking for 15–20 min at 160 rpm, the samples were centrifuged at 5000 rpm, 40°C, 10 min. The supernatant was besieged in a 3-fold volume of 96% ethanol in the presence of a 3M solution of sodium acetate pH 5.2, the samples were left overnight at -20°C. DNA preparations were washed with 70% solution of ethanol. The obtained DNA preparations can be stored at -20°C for a month. DNA / RNA and intact DNA preparations were analyzed in a 2% agarose gel containing 0.5 μg / ml of ethidium bromide. Electrophoresis was carried out for 1 hour at 100 V, the gel was photographed in passing ultraviolet rays.The expression of the matrix RNA of the studied p53 and Bcl-2 genes was determined by a semi-quantitative method of polymerase chain reaction (PCR) with reverse transcription. The amplification reaction was carried out according to the following scheme: denaturation - 94°C, 10 c; annealing - 10 c; synthesis - 72 °C, 20 c. As an internal control to assess the amount of RNA taken in the reaction GAPDH expression was determined. Reverse transcription polymerase chain reaction products were analyzed by electrophoresis on a 2% agarose gel with the addition of 0.5 μg / ml of ethidium bromide.Reverse transcription (RT-PCR)Total RNA was obtained using a kit for extracting RNA from tissues “EZ1 RNA TissueMiniKit” (for 48 samples), produced by “InterlabService”, (Moscow, Russia). The kit “REVERTA-L” (Russia) was used to obtain extracellular DNA on an RNA matrix. The reverse transcription reaction was performed according to the manufacturer's protocol. Primers for the p53 and Bcl-2 genes were used. The primers of the reference GAPDH gene were used as a control. PCR was performed on a “BioRad” (the USA) amplifier. The PCR products were stored at -20°C.Electrophoresis of PCR products was performed in 2% agarose in TAE buffer, 100 V, 1 h. Then the agarose gel was stained for 15 min in ethidium bromide solution (0.5 μg / ml). The results of electrophoretic analysis in the gel were visually observed through the passing ultraviolet rays on the “Bio-Rad” (the USA) transilluminator. The agarose gel was scanned after electrophoresis on a densitometer which analyzes the luminescence intensity of PCR products. The resulting image of the bands was processed using a computer densitometer (Gel-Pro-Analizer 4.0).
3. Results and Discussion
- Today, the treatment of hysteromyoma is the most difficult and debatable problem [4]. The choice of treatment method is determined by many factors: the features of pathogenesis, the form and rate of tumor growth, age, absence or presence of children in a woman, etc. [1,4]. The task of therapy is to remove a tumor (surgical treatment) or to inhibit tumor growth and to regress the tumor (conservative treatment). 180 patients of different age categories with various forms of hysteromyoma were examined. The patients were divided into 3 groups: 1 - simple uterine myomas (65 patients); 2 - progressive (fast-growing) uterine myomas (58 patients); 3 - symptomatic myoma - menstrual disorder, bleeding symptom (57 patients). Healthy donors (20) were as a control group. The criteria of sick women inclusion in the study were: the presence of hysteromyoma in women of reproductive and premenopausal age with different development of the clinical symptoms of the disease. The use of modern treatment methods does not always provide a high therapeutic effect and the problem of the disease recurrence is persisted. The search for new methods of diagnostics and treatment based on an in-depth research of the insufficiently studied issues of the uterine proliferative diseases pathogenesis has been going on.Particular importance in these issues solution belongs to the study of this pathology occurrence from the standpoint of identifying emerging disorders of proliferation and apoptosis. An important role of the p53 gene in the origin and development of hysteromyoma which protects the body from mutant cells was noted. The key function of p53 is to stop the cell cycle and to increase the duration of the premitotic phase for the implementation of reparation synthesis. Activated p53 protein is able to influence the regulation of the cell cycle through a number of mechanisms [10]. Mutations of the p53 gene allow the cells with damaged DNA to maintain mitotic activity. Incompleteness of apoptosis explains the different sizes and different degrees of nodes maturity, the benign nature of the tumor, non-invasive and slow tumor growth. Bcl-2, in turn, suppresses apoptosis of cells. Table 1 presents the sequence of primers for the p53 and Bcl-2 genes.
|
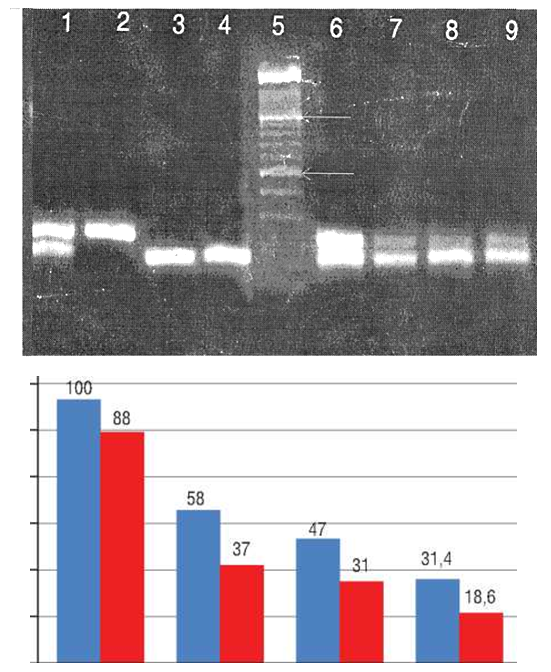 | Figure 1. Expression of p53 and bcl-2 genes in patients with progressive (fast-flowing) hysteromyoma (blue columns – the Bcl-2 gene, red columns – the p53 gene) |
 | Figure 2. Expression of the p53 and bcl-2 genes in patients with simple hysteromyoma ((blue columns – the Bcl-2 gene, red columns – the p53 gene); x2 = 4.84, p = 0.0185, OR = 6.84; 95% CI (34-26.6) |
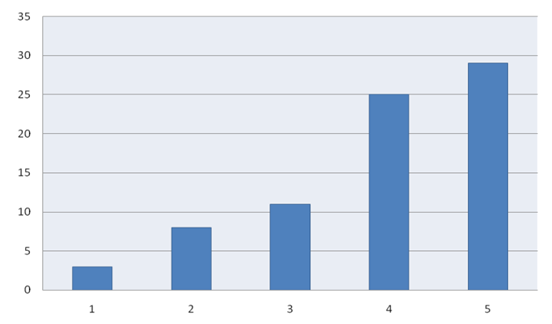 | Figure 3. Expression of the Bcl-2 gene in the treatment of various hysteromyoma. (x2 = 5.62, p = 0.018, OR = 7.91; 95% CI (44-36.90) |
4. Conclusions
- The study of molecular genetic processes of violation of the apoptosis start in women with hysteromyoma has a very important practical and theoretical importance, allows to expand the possibilities of diagnostics, prognosis of the clinical course and the effectiveness of therapy.There was a decrease in the expression of the Bcl-2 gene and an increase in the expression of the p53 gene in women with simple hysteromyoma. As a result of the Bcl-2 gene blocking via p53, the mechanism of apoptosis is triggered and prevents further cell division and the growth of myomatous nodes. There was an increase in the expression of the Bcl-2 gene and a decrease in the expression of the p53 gene in women with fast-growing hysteromyoma. Increased expression of the Bcl-2 gene is a marker for rapid growth of hysteromyoma and the ineffectiveness of the conducted therapy.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML