-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2019; 9(9): 337-341
doi:10.5923/j.ajmms.20190909.06

Influence of Polymorphism C3435T MDR1 Gene to Methotrexate Resistance in Patients with Rheumatoid Arthritis
Nargiza Abdurakhmanova, Khalmurad Akhmedov
Department of Internal Disease #3, Tashkent Medical Academy, Tashkent, Uzbekistan
Correspondence to: Nargiza Abdurakhmanova, Department of Internal Disease #3, Tashkent Medical Academy, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In our study, 120 patients with rheumatoid arthritis and 30 practically healthy volunteers took part. Polymorphism of the C3435T gene was determined using the polymerase chain reaction (PCR), which was carried out in a 7500 Fast Real Time PSR Systems (USA) amplifier. Results: According to the results of our studies, methotrexate resistance was detected in patients with CC genotype C3435T polymorphism of the MDR1 gene. In contrast, carriers of TT genotype showed high sensitivity to treatment and low disease activity. The interrelation of the C3435T polymorphism of the MDR1 gene with the presence of resistance to methotrexate treatment in patients with RA was established. Conducting genotyping of patients with rheumatoid arthritis before the appointment of methotrexate makes it possible to predict the presence of resistance to this drug.
Keywords: MDR1, C3435T, Methotrexate, Rheumatoid arthritis, Genotype, Polymorphism
Cite this paper: Nargiza Abdurakhmanova, Khalmurad Akhmedov, Influence of Polymorphism C3435T MDR1 Gene to Methotrexate Resistance in Patients with Rheumatoid Arthritis, American Journal of Medicine and Medical Sciences, Vol. 9 No. 9, 2019, pp. 337-341. doi: 10.5923/j.ajmms.20190909.06.
1. Introduction
- Rheumatoid arthritis (RA) is an important medical and social problem, its relevance is due to the progressive course of the disease, the severity of the musculoskeletal system, the high frequency of lesions of people of working age, the early occurrence of reduced functional abilities, loss of professional and social skills, difficulty of physical and psychological adaptation patients to motor impairment, significant disability, leading to huge economic losses. The disease occurs in people of working age and leads to rapid disability and reduced life expectancy of patients. [7]Immunosuppressive and biological therapies are known to suspend the development of patient disability, which may occur due to the progression of joint destruction and systemic manifestations. However, it is far from all cases that clinical remission can be achieved with basic therapy. According to some estimates, from 50 to 90% of adverse pharmacological responses are determined by the genetic characteristics of individuals [5]. The study of individual genetic differences underlying the variability of the body's response to a particular drug is of paramount importance for the optimization of pharmacotherapy.Today, the main task of clinical rheumatology is the optimization of pharmacotherapeutic approaches to the treatment of patients, and thus the improvement of the outcome of the disease, the reduction of the level of disability of patients. Pharmacogenetics is a section of clinical pharmacology and clinical genetics, studying the genetic characteristics of the patient, affecting the pharmacological response. [11] Identification of the corresponding allelic variant that causes a change in the pharmacokinetics of the drug, allows you to adjust therapy (dose, frequency and route of administration, replacement of drugs, etc.) and increase its effectiveness and safety. Identification of allelic variants (genotyping) of some of these genes is widely used abroad in clinical practice for the individualization of pharmacotherapy. One of these genes is MDR1, which is responsible for resistance to various drugs, and its polymorphism can affect the pharmacokinetics of many cytostatics, including methotrexate, which is included in the “gold standard” of RA treatment [4,6,10]. The MDR1 gene, encoding glycoprotein-P, has polymorphism. The most clinically significant is considered polymorphism C3435T gene MDR1.Evaluation of the polymorphism of the MDR1 gene in patients with RA is a topical issue in rheumatology, as it remains controversial about its impact on the effectiveness of therapy, duration of remission and prognosis of RA, as well as on the metabolism of methotrexate. The study of the polymorphism of the MDR1 gene can be an important link in the development of a personalized approach to the treatment of RA.
2. Materials and Methods
- The study was conducted at the 3-City Clinical Hospital No. 3 in the rheumatology department of the city of Tashkent. The study was conducted in 120 patients (107 women, 13 men, 18-65 years old) with RA. The control group consisted of 30 healthy volunteers, without the burdened rheumatological history. RA was diagnosed according to the criteria of the American College of Rheumatology (ACR), and disease activity was calculated using the DAS28 calculator. All patients who participated in our study were prescribed basic therapy (methotrexate in monotherapy at a dose of 7.5-15 mg / week) and patients were monitored for 6 months.For the genotyping of the C3435T MDR1 polymorphism of the gene, venous blood was taken in a volume of 3 ml from patients during their hospitalization in the rheumatology department and stored in EDTA tubes. Genomic DNA was extracted from blood samples using RIBO prep reagents (AmpliSens, Russia). For genotyping, a reagent kit was used to determine the C3435T polymorphism of the MDR1 gene (SINTOL, Russia). Polymorphism of the C3435T gene was determined using the polymerase chain reaction (PCR), which was carried out in a 7500 Fast Real Time PSR Systems amplifier (Applied Biosystems USA).Statistical analysis. The results were subjected to static processing using the computer program EXCEL and STATISTICA 6.0. A comparative analysis was performed using the standard Pearson X2 test and the two-sided Fisher test, where p <0.05 was considered statistically significant.
3. Results
- In our study, 120 patients with RA and 30 healthy volunteers without rheumatological history were studied. All patients took methotrexate, after a six-month period, the patients were divided into three groups: 35 (29.1%) patients received methotrexate in a dose of 7.5-20 mg / week in a clinical remission that lasted for 6 months or more. They were allocated by us to the I group. In 48 (40%) patients, clinical remission was observed for 3-6 months, on the basis of which we identified them in group II. And in the remaining 37 (30.9%) patients, clinical remission in spite of an increase in the dose of methotrexate for every 5 mg per month lasted no more than 3 months, these patients were allocated to us in group III.The study of the activity of the disease according to the DAS28 calculator before (1 day) the beginning of inpatient treatment, and then every month for 6 months in three groups was as follows: in the first group, the average activity indicators of the process were DAS28 <2.6 for 6 months. In the second group, during 6 months, the activity index averaged DAS28=4.2 (p <0.05). In the third group, despite the treatment carried out according to the recommendations of EULAR, in 30,9% of patients the activity of the process remained high DAS28>5.1.The data obtained indicate that the clinic of RA is characterized by variability of the course with different duration of periods of exacerbation and remission, which are apparently associated with differences in the therapeutic response of patients at the pharmacological level.All patients were genotyped for the polymorphism of the C3435T MDR1 gene. The results of genotyping are shown in fig.2.
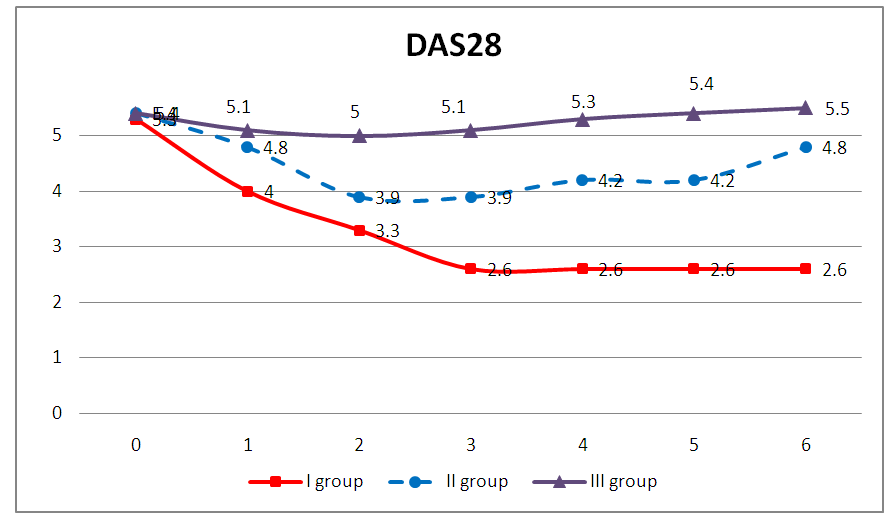 | Figure 1. Dynamics of activity indicators of RA according to the DAS28 in the studied sample of patients for 6 months |
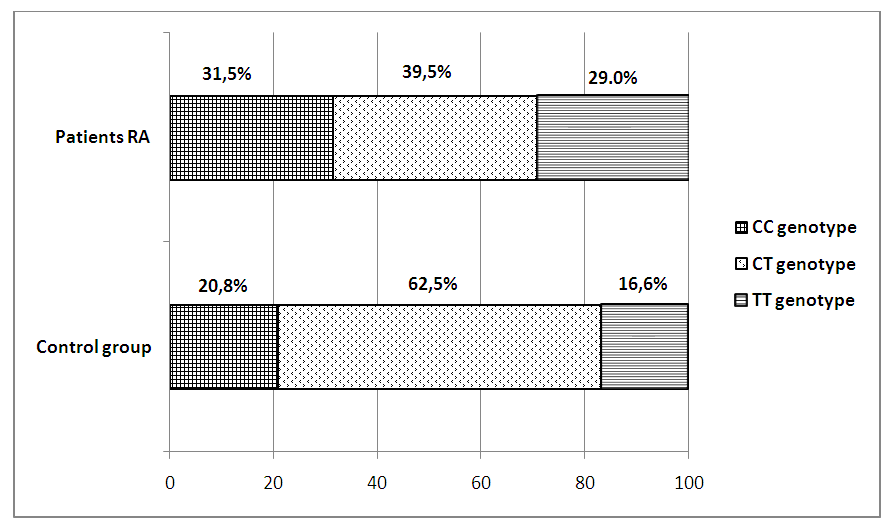 | Figure 2. The percentage distribution of the genotypes of the polymorphism of the C3435T MDR1 gene in patients with RA and in the control group |
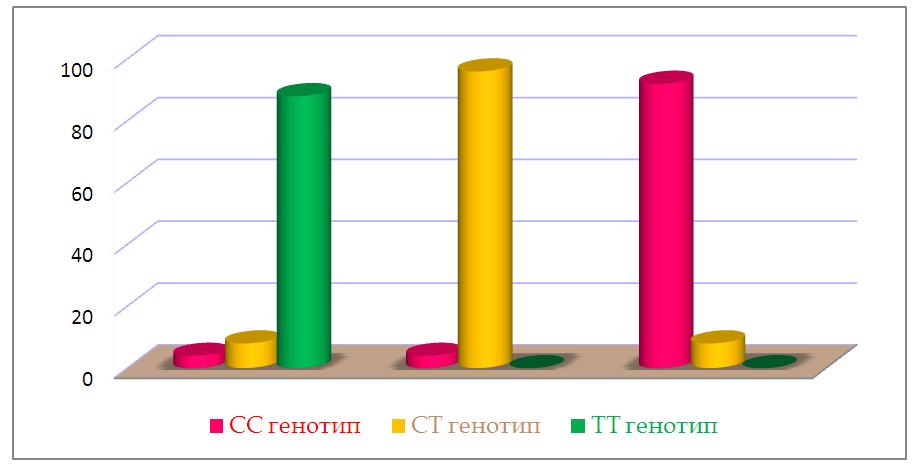 | Figure 3. Distribution of genotypes of the C3435T polymorphism of the MDR1 gene in RA patients in three groups |
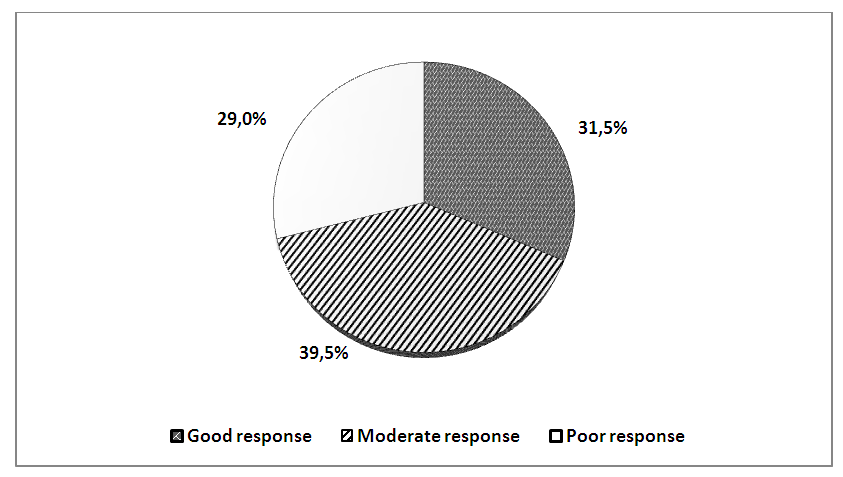 | Figure 4. Percentage distribution of RA patients depending on the drug response to treatment with methotrexate |
4. Discussion
- As mentioned above, drug resistance is one of the important factors affecting the effectiveness of RA therapy. Individualization of pharmacotherapy, which is engaged in pharmacogenetics, consists of identifying polymorphic markers associated with a change in the body's response to drugs, developing methods for genotyping patients and introducing this approach into practical medicine. Some researchers suggest [1] that there is an association of MDR1 gene polymorphisms with the efficacy and safety of many drugs. A number of researchers (Takatori et.al., Sharma et al.) Conducted a cohort study of C3435T with the isoform of the MDR1 gene (ABCB1) in RA patients and concluded that TT genotype is associated with poor susceptibility to treatment of MTX, and CC genotype is associated with a good response to treatment. On the contrary, other researchers (Chen et.al.; Drozdzik et.al.; Pawlik et.al.) conducted the same studies and concluded that CC genotype is associated with immunity to treatment of MTX in RA.Despite the large number of studies devoted to the study of the polymorphism of the gene MDR1, the results remain controversial, which caused us some interest. According to our data, there were no significant differences in the distribution of alleles of the C3435T polymorphism of the MDR1 gene in RA patients and conditionally healthy ones. But when comparing genotypic variants, we found differences: healthy CC and mutant TT genotypes were more common in patients, and heterozygous CT genotype was more common in healthy ones.According to the results of genotyping, we established the relationship of the C3435T polymorphism of the MDR1 gene with the presence of resistance to MTX and disease activity. We identified three groups of respondents to treatment with this drug. Good responders - owners of the mutant TT genotype showed a good clinical response to treatment with methotrexate, as well as low disease activity (Das28 <2.6). Bad responders (patients with CC genotype) had resistance to methotrexate, even with an increase in the dose of the drug, clinical remission or low activity (Das28> 5.1) did not reach the disease. The moderate responders were CT carriers of the genotype of the C3435T polymorphism of the MDR1 gene, which showed an average drug response to MTX, and the disease activity was moderate (DAS 28 3.2–5.1).
5. Conclusions
- Genetic studies of the C3435T polymorphism of the MDR1 gene in RA patients revealed their interrelation with resistance to MTX, as well as disease activity during treatment with this drug.In RA, there is a different clinical response to basic therapy (metatrexate). So, in 29.2% of cases, the duration of clinical remission (DAS28 <2.6) is observed for 6 months or more, in 40% of cases, 3-6 months within DAS28 = 3.2-4.6, and also in 30, 8% of cases - not achieved complete clinical remission.The clinical efficacy of basic therapy for RA depends on the polymorphism of the MDR1 gene. Thus, the TT genotype C3435T isoform of the MDR1 gene is associated with the most significant clinical response to basic therapy. The presence of CC genotype of the MDR1 gene in RA is associated with a negative clinical effect on methotrexate, since the clinical response remains within DAS28> 5.1, which indicates the presence of resistance to this drug. The owners of CT genotype demonstrated a decrease in activity from high to medium numbers (DAS28 = 3.2-4.6), which indicates a lack of effectiveness of the drug.A personalized approach in RA allows to predict methotrexate resistance in patients with RA when performing genotyping of the C3435T polymorphism of the MDR1 gene before prescribing this drug.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML