-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2019; 9(7): 262-269
doi:10.5923/j.ajmms.20190907.08

Metabolic Effectiveness of Laparoscopic Sleeve Gastrectomy in Morbid Obesity
F. G. Nazirov, Sh. Kh. Khashimov, U. М. Makhmudov, Z. R. Khaybullina, O. D. Tuychiev
Department of Endovisual Surgery, State Institution “Republican Specialized Scientific-Practical Medical Centre of Surgery named after Academician V.Vakhidov”, Tashkent, Uzbekistan
Correspondence to: U. М. Makhmudov, Department of Endovisual Surgery, State Institution “Republican Specialized Scientific-Practical Medical Centre of Surgery named after Academician V.Vakhidov”, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Metabolic effectiveness of laparoscopic sleeve gastrectomy (LSG) have been evaluated in 3 month after surgery according plasma lipidomic profile, fasting glucose, levels of proinflammatory cytokines (interleukin-6 (IL-6), tumor necrosis factor alpha (TNF-alpha), C-reactive protein (CRP), as well as oxidative stress marker malondialdehyde (MDA), vasculo endothelial growth factor (VEGF) at patients with morbid obesity (MO). It was established, that LSG is not accompanied by activation of systemic inflammation in the early postoperative period and leads to decreasing in the initially elevated levels of pro-inflammatory factors in serum at 3 months after the intervention. The results indicate a positive effect of LSG on the elimination of inflammation and metabolic disorders in obese patients. LSG is efficient in reducing the amount of visceral fat by reducing body mass index and waist circumference (WC) at 3 months after the intervention. LSG reduces cardiometabolic risk due to normalization of the lipid profile and indicators of oxidative stress (OS) in blood, helps to optimize the paracrine and endocrine functions of adipose tissue by reducing inflammation in it.
Keywords: Laparoscopic sleeve gastrectomy, Inflammation, Metabolic effectiveness
Cite this paper: F. G. Nazirov, Sh. Kh. Khashimov, U. М. Makhmudov, Z. R. Khaybullina, O. D. Tuychiev, Metabolic Effectiveness of Laparoscopic Sleeve Gastrectomy in Morbid Obesity, American Journal of Medicine and Medical Sciences, Vol. 9 No. 7, 2019, pp. 262-269. doi: 10.5923/j.ajmms.20190907.08.
Article Outline
1. Introduction
- Obesity is the most important component of metabolic syndrome, an independent risk factor for a number of socially significant diseases - arterial hypertension, ischemic heart disease (IHD), type 2 diabetes, and a risk factor for premature death [8,29]. It is certain, that with a body mass index (BMI) of more than 35 kg/m2 in combination with a waist circumference (WC) of more than 90 cm and a triglyceride (TG) level of more than 2,3 mmol/l, the risk of cardiovascular diseases (CVD) increases 20 times [24]. At the same time, “metabolically healthy individuals” are found among obese people, and a BMI without consideration WC cannot be criteria for the CVD risk [36]. In a study of 15547 patients with IHD, the highest risk of death was observed in patients with the normal BMI, and increased WC [7]. The magnitude of WC and all-cause mortality are linearly dependent [15]. WC reflects the amount of visceral fat. According to M.R. Salazar et al. (2014), an increase in WC, more than 90 cm, corresponds to visceral adipose tissue, having an area of more than 130 cm2, and the excess of this value is combined with metabolic disorders [35]. However, it is not precisely established that exactly obesity causes comorbidity and increases cardiometabolic risks - high visceral fat, adipocyte dysfunction, elevated levels of TG and free fatty acids, impaired adipo-cytokines secretion, low-intensity inflammation, oxidative stress (OS) or other factors [21].Possibly, cardiometabolic risk in obesity is associated with the condition of the functional activity of visceral adipose tissue and the intensity of inflammation in it [34]. Visceral adipose tissue has a high metabolic activity and is considered as a part of endocrine system, that produces leptin, apelin, adiponectin, angiotensinogen, insulin-like growth factor, insulin-binding protein, monobutyrin, TNF-alpha, IL-6, plasma activator of plasminogen-1 inhibitor (PAI-1), resistin, lipoprotein lipase, acetylation stimulating protein, cholesterol ester transfer protein, retinol-binding protein, estrogens [5]. When TG are accumulated in adipocytes, macrophage cells start to infiltrate the adipose tissue, meanwhile activated macrophages, as well as adipocytes, are considered as producers of pro-inflammatory cytokines — TNF-alpha and IL-6 [4], triggering and maintaining low-intensity inflammation [21]. Macrophages of adipose tissue are represented by two phenotypes: M1 and M2. M1 phenotype is “activated” macrophages that blocks adipocyte differentiation, as a result, occurs their hypertrophy, adipokines secretion is deteriorated and ectopic leptin cumulates in the liver, muscles and other tissues. M2 phenotype is an anti-inflammatory phenotype of macrophages, it is formed from M1 under the action of a PPAR gamma agonist (peroxisomal proliferative factor receptor agonist) [12]. Adipocytes and macrophages of adipose tissue (AT) produce inflammatory cytokines, including TNF-alpha, IL-1, PAI-1, monocytic chemoattractant protein-1 [5]. Adipokines, primarily leptin and adiponectin, are also involved in the regulation of the inflammatory process [10,25,36]. Adipose tissue dysfunction and adipocytokine dysregulation, associated with inflammation, consequently makes for insulin resistance and the formation of metabolic disorders [38].In this regard, the study of the intensity of inflammation in obesity and weight loss in dynamics after surgical bariatric intervention is of considerable interest.Of all the methods of treating obesity, surgical is the most effective [26]. A meta-analysis of surgical treatment of obesity, in which studied effectiveness of weight loss (89 studies), incidence (134 studies) and complications (128 studies) of surgical treatment of obesity, showed that weight loss after surgery is 20-30 kg in patients with an initial BMI of more than 40 kg/m2 and is resistant (up to 10 years). Weight loss allows controlling comorbid conditions in obesity [27]. Data from a randomized trial of the effectiveness of laparoscopic gastric bypass showed that weight loss by 13.6% in 5 years after surgery was accompanied by a decrease in cardiometabolic risk markers: high-density lipoprotein (HDL), cholesterol (CH), TG, glucose [33]. After bariatric surgery, manifestations of type 2 diabetes are stopped in 86.6% of cases [19]. The most effective weight loss is achieved by using biliopancreatic and duodenal bypass, however, after these operations, physiological digestion is disturbed, vitamins deficiency and malabsorption were developed [33,41], and the intensity of systemic inflammation syndrome does not decrease [23].
2. Aim of the Study
- The purpose of this study was to evaluate metabolic outcomes of the LSG, as well as the intensity of systemic inflammation and oxidative stress at patients with morbid obesity (MO) after bariatric surgery – LSG.
3. Materials and Methods
- The study involved 37 patients (29 female and 8 male) with MO, operated in State Institution “RSSPMC of Surgery named after acad. V.Vakhidov” in the period from 2016 to 2019. All individuals were non-smoking, the average age was 36,5±2.4 years, and BMI = 51,2±2,3 kg/m2. The control group consisted of 10 female volunteers aged 38.4 ± 1.9 years, without obesity (BMI = 23.4 ± 0.3 kg/m2), having an WC = 76.1 ± 1.0 cm. Minimally invasive surgery – LSG – was performed to 37 patients. Interventions were performed by the OR-1 endoscopic surgical complex and the toolset of Karl Storz GMBH & CO.KG (Germany). During surgical endovisual intervention were used: the Force Triad energy platform with LigaSure technology from Covidien (USA), the Harmonic G11 ultrasonic scalpel (Johnson & Johnson, USA), and the endoscopic stapling-cutting device from Ethicon Endo Surgery (Johnson & Johnson, USA). This endovisual intervention is considered as a restrictive bariatric surgical procedure. The technique of laparoscopic surgery was to remove most of the stomach, located along gastric curvature major, with preservation of the cardiac sphincter and pylorus and the formation of a narrow gastric tube with a volume of 60-150 ml, located along the gastric curvature minor.
3.1. Operational Technique
- LSG was performed under general endotracheal anesthesia. The operation is usually involved three surgeons. The patient was placed in the position of Trendelenburg (with an elevated head end), while the legs were separated so that the operating surgeon can be placed between them.As a rule, used 5-port access. A 30° optics was inserted through a trocar placed on 10–12 cm of supraumblical area. The remaining trocars were inserted on approximately the same horizontal line. On the left midclavicular line, a 12-mm trocar was installed for the instrument in the surgeon’s right hand, on the contralateral same area was placed a 12-mm trocar for the surgeon’s left hand and the subsequent stapling device, on the left anterior axillary line - the 5-mm trocar for the assistant’s instrument. The next trocar was inserted for use of the hepatic retractor, it was placed subxiphoidal along the middle or right anterior axillary line.Using the harmonic scalpel Harmonic G11 (Johnson & Johnson, USA) and LigaSure Atlas of ForceTriad energy platform (Covidien, USA), a full mobilization of the stomach along the curvature major was performed: from the pyloric part to the angle of the Hiss, dissected the gastrocolic ligament, were cut short gastric vessels, completely mobilized the fundus of the stomach on the back wall (Figure-1).
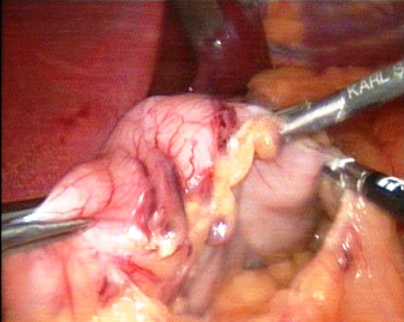 | Figure 1. Intraoperative photo. Stomach mobilization along greater curvature (gastric curvature major) |
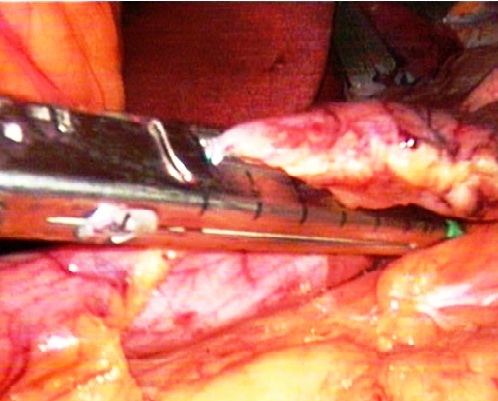 | Figure 2. Intraoperative photo. Stapling device during firing |
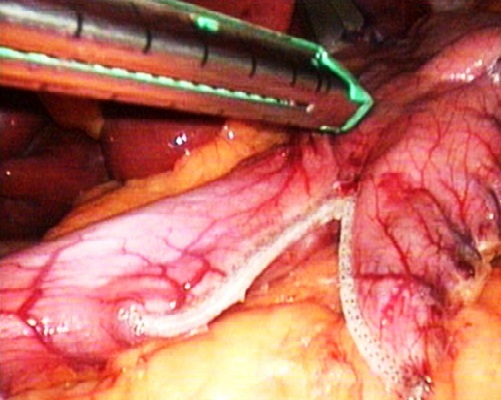 | Figure 3. Intraoperative photo. The stage of forming sleeve with firing and excising device |
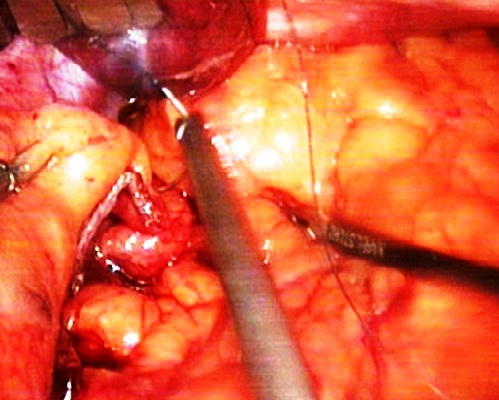 | Figure 4. Intraoperative photo. Maintaining the staple line |
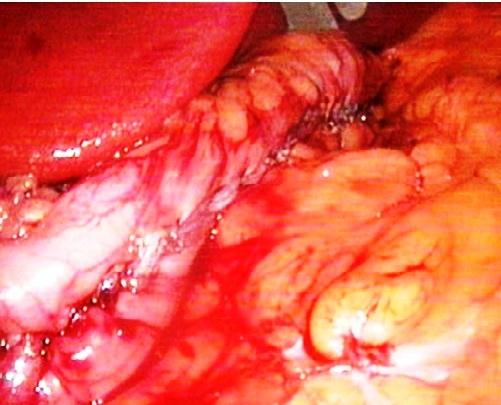 | Figure 5. Intraoperative photo. Final appearance of gastric sleeve |
3.2. Laboratory Assessment
- Data of plasma lipid profile: total CH, TG, HDL, very low-density lipoproteins (VLDL), as well as C-reactive protein (CRP), fasting glucose, uric acid (UA), total protein (TP), and albumin was obtained from an automatic biochemical analyzer "VITROS-350" (Ortho Clinical Diagnostics, USA). Low-density lipoprotein (LDL) was calculated by Frivald’s: LDL = CH-(HDL-TG/2.2), and atherogenic index (AI) - according to Klimov manner: AI = (CH - HDL)/HDL. Total blood count includes the amount of WBC, monocytes (M), lymphocytes, that performed an automated hematology analyzer BC 5800, Mindray (China). Serum cytokines: IL-6, TNF-alpha, as well as vasculoendothelial growth factor (VEGF), were determined by ILISA kits of "Vector-BEST" (Russia) on the ST-360 IFA analyzer (China). Malon dialdehide (MDA) was determined by Ohkawa in the modification of Al-Gayyar (2007). Catalase activity was investigated according to M.A. Korolyuk. (1988).
3.3. Statistical Methodology
- Statistical processing of amount data was performed using ANNOVA test, pare t-Test by the Microsoft Excel software package. The data are presented as M±m, the differences were considered significant at a level of p <0,05.
4. Results and Discussion
- At patients with MO metabolic status before surgery includes increasing TG level 2,7 times vs control group, increasing CH level (p<0,05), decrease HDL level (p<0,05), increasing UA level in 2,5 times vs to control group. The level of CRP, IL-6 and TNF-alpha in the blood was increased by 3,5, 2,7 and 5,3 times vs to control group, respectively. WBC number was within the reference interval (4-9*109/l), however, it was significantly higher (p<0,05) vs the control group. Also in patients with obesity, there was an increase in monocytes by 2.0 times relative to the control group (Table 1).
|
5. Conclusions
- Morbid obesity is characterized by changes in the serum lipid spectrum, hyperuricemia, and inflammation with increasing serum levels of IL-6, TNF-alpha, CRP. At the same time, a weak expression of oxidative stress and normal endothelial function are typical in morbid obesity.Restrictive bariatric surgery - laparoscopic sleeve gastrectomy helps to reduce the intensity of systemic inflammation via reduction the plasma CRP level to the reference range, decreased IL-6 and TNF-alpha in 1.6 and 2.2 times from data before surgery.Laparoscopic sleeve gastrectomy contributes to effective weight loss, decreasing WC, normalizing lipid and carbohydrate metabolism parameters, reducing asymptomatic hyperuricemia and oxidative stress.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML