-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2019; 9(7): 236-241
doi:10.5923/j.ajmms.20190907.03

Exercise-Induced Increase in Progesterone does not Change FVC and FEV Values in Non Asthmatic Individuals
Messan Folly1, Donouvi Jéronime1, Lawani Mohamed Mansourou2
1Laboratory of Respiratory, Hormonal and Gerontological Explorations of the Sportsman, National Institute of Youth, Physical Education and Sport (INJEPS), University of Abomey-Calavi, Cotonou, Republic of Benin
2Laboratory of Biomechanics and Performance (LABIOP), National Institute of Youth, Physical Education and Sport (INJEPS), University of Abomey-Calavi, Cotonou, Republic of Benin
Correspondence to: Messan Folly, Laboratory of Respiratory, Hormonal and Gerontological Explorations of the Sportsman, National Institute of Youth, Physical Education and Sport (INJEPS), University of Abomey-Calavi, Cotonou, Republic of Benin.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

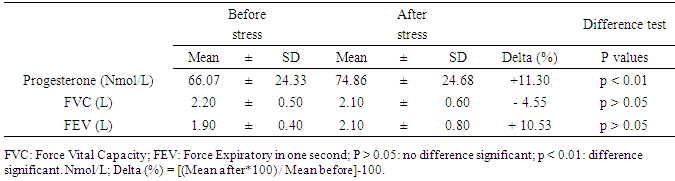
Background: Progesterone hormone levels increase as a result of different exercise modalities in non-menopausal sportswomen. Progesterone is also involved in dilating the airways when exercise-induced bronchospasm (EIB) occurs in sensitive women. Objective: To determine the effect of elevated exercise-induced progesterone levels on the respiratory airways of non-EIB-susceptible women. Methods: Eleven pre-menopausal girls were recruited. During the luteal phase of their menstrual cycles, blood and respiratory explorations were performed before and after undergoing a comprehensive stress test performed on a cycle ergometer. Progesterone was assayed, and FVC and FEV were determined. Results: The average value of serum progesterone observed after exercise (74.866 ± 24.68 Nmol / L) was significantly (p < 0.05) higher than that recorded (66.072 ± 24.33 Nmol / L) before exercise. The average values of FVC (2.20 ± 0.5 L) and FEV1 (1.90 ± 0.4 L) observed before exercise and those recorded after exercise (2.10 ± 0.6 and 2.10 ± 0.8, respectively), did not significantly differ (p > 0.5). Conclusion: Progesterone, a bronchodilator hormone, does not influence the respiratory airway in subjects that are not sensitive to bronchospasm, even though the stress test induces a high level of this hormone.
Keywords: Menstrual cycle, Progesterone, FEV, FVC, bronchospasm, Stress test
Cite this paper: Messan Folly, Donouvi Jéronime, Lawani Mohamed Mansourou, Exercise-Induced Increase in Progesterone does not Change FVC and FEV Values in Non Asthmatic Individuals, American Journal of Medicine and Medical Sciences, Vol. 9 No. 7, 2019, pp. 236-241. doi: 10.5923/j.ajmms.20190907.03.
Article Outline
1. Introduction
- Asthma is a multi factorial condition resulting from a combination of predisposing congenital factors (hereditary factors) and environmental factors (allergens, pollen, inhaled air pollution, etc.). Asthma is defined as a condition characterized by respiratory discomfort with dyspnoea or paroxysmal wheezing, most often during exhalation, indicating a sudden decrease in the size of the bronchi, which is gradually associated with oedema. As a non communicable disease, bronchospasm is a public health problem with a prevalence ranging from 4% to 20% in the general population and from 48% to 55% or more in the sports population [1-6]. This state of affairs is explained by the high levels of exercise and training to which athletes are compelled. To this end, the quantity of inhaled and exhaled air is very important and leads to spasm of the bronchi in sportsmen, although it is noted that before the pubertal period, the symptoms and prevalence of asthma observed in girls and boys were not significantly different. However, epidemiological studies in sedentary women have shown that the prevalence of bronchospasm observed in sporting women is significantly higher than that observed for male athletes [7-11]. In addition, self-administered surveys of athletes at the 1996 Summer Olympics revealed that the prevalence of bronchospasm observed in women athletes (19.9%) was higher than that reported for male athletes (14.3%) [11]. Finally, during the 1998 Olympic Winter Games, this trend was confirmed, especially as the prevalence of EIB among sportswomen was 35.4% compared with 13.2% among male athletes [11-13]. In summary, how can we explain that in the post-pubertal period, the prevalence of asthma observed in girls is higher than that observed in boys? The answer to this concern comes from the fact that the menarche (first menstrual cycle) and the following cycles constitute an important physiological phenomenon that differentiates young girls from the young boys. Indeed, the menstrual cycle is characterized by two phases, follicular and luteal, separated by ovulation in the middle of the cycle. From the follicular phase to the luteal phase, the oestradiol concentration gradually increases to its maximum before ovulation, while the progesterone level remains low but will increase from ovulation until the end of the cycle at which women are exposed during menstrual cycles can be partly explained by [14]. This fluctuation of the sex hormones, particularly estrogen and progesterone, is not without consequence to the reactivity of the respiratory airways according to the phases of the menstrual cycle. Indeed, for approximately 30 years, studies have highlighted the influence of sex hormones on the respiratory airways of healthy sedentary women [15-17]. However, the link between sex hormones and bronchospasm is not very clear [18]. The physiological mechanisms that explain the cyclic respiratory variations occurring during the menstrual cycle are different depending on whether the subject is athletic and/or sensitive to bronchospasm [19]. However, Stanford et al. (2006) [20] showed that in asthmatic subjects, the luteal phase of the menstrual cycle is a major determinant of exercise-induced asthma. As a result, EIB-sensitive subjects showed a significant decrease in oestrogen and progesterone levels following a stress test that resulted in an exacerbation of asthma symptoms or a decrease in lung function [21-23]. This concept has only been confirmed by the work of Messan et al. (2017) [24], who showed that in professional EIB-sensitive cyclists, circulating catecholamine attenuates the effects of exercise-induced bronchospasm [24]. Under these conditions, catecholamine concentrations are relatively lower in these cyclists because they are used to dilate depressed airways. Other studies have shown that high levels of progesterone can exacerbate not only asthma symptoms [25,26] but also bronchial responsiveness [27], eventually even inhibiting the development of Th-1 lymphocytes and improving the production of Interleukin-4 [28]. However, the different effects of the modalities of exercise on the concentration levels of progesterone have not been sufficiently evoked in normal subjects without bronchospasm. In fact, among younger Nigerians who are less trained, a six-week programme of moderate physical training was able to induce an alteration in the concentration levels of certain hormones [29]. Similarly, an isolated exercise does not alter the level of estrogen, while chronic exercise can inhibit its release [30]. During the luteal phase, exercise causes a significant increase in the concentration of progesterone [31-33]. Based on the hypothesis of a high concentration of progesterone in subjects not sensitive to EIB at the end of an exercise, would the post-exercise values of the FVC (Force Vital Capacity) and the FEV (Force Expiratory in one second) vary significantly from their pre-exercise values? Therefore, to test this hypothesis during the luteal phase in non-asthmatic and untrained students, this study aims to 1) assay the progesterone levels in two blood samples taken before and after exhaustive exercise; 2) explore the state of pulmonary function by determining the FVC and FEV before and after exhaustive exercise; and 3) compare the progesterone, FVC and FEV concentrations observed before exercise to those recorded after exercise.
2. Materials and Methods
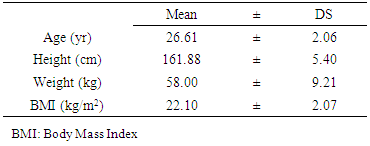
- The study was conducted at the National Institute of Youth, Physical Education and Sport (INJEPS) of Porto-Novo in Benin, West Africa. Eleven healthy girls with a regular menstrual cycle of 28 plus or minus two days voluntarily agreed to participate in this study (age: 20.61 ± 2.06 years, height: 161.88 ± 5.40 cm and body mass: 58.00 ± 9.21 kg). After being informed about the purpose and potential risks of the study, all participants signed the informed consent form. The experimental protocol was approved by the Sectorial Scientific Committee of INJEPS. Exercise-induced bronchospasm was tested in girls by a post-exercise fall of at least 10% in FEV compared with its pre-exercise value. At the end of this diagnosis those whose post-exercise FEV declined by at most 10% were declared not sensitive to bronchospasm and were retained in the study. Individuals who did not meet this criterion were excluded from the study, as were those who were sensitive to asthma symptoms. Girls who have a regular menstrual cycle of 28 plus or minus two days were also selected. The study was conducted in accordance with the Helsinki Declaration. The prior medical visits to which the girls were subjected showed that they were all in good health, not pregnant and under no medical treatment. The subjects also had not taken oral hormonal contraceptives for at least six months before the start of the study. All girls enlisted in this study did not have an intensive sports practice.
2.1. Study Design and Protocol
- In this study, the experiments were conducted at D+21 and D, as the day when the menses of each subject begin. D+21 corresponds to the 22nd day of menses, and on this day, the affected student visits the laboratory within 9 hours for anthropometric measurements. The student is then subjected to passive rest on a chair for 10 minutes, after which blood and respiratory functional explorations were performed by a qualified nurse. After an exhaustive triangular exercise test, each subject was immediately submitted to a blood test. The functional exploration occurred 5 minutes after the effort.
2.2. Blood Sampling and Analysis
- On the 22nd day of their menstrual cycle, each subject visited the laboratory between 9 and 10 o'clock. Pre-and post-exercise blood samples are drawn by a qualified medical practitioner established in the open for the purpose of each procedure. A three-way valve with an injection valve and a 25 cm extension allowed blood sample sterility without painful embarrassment or hindrance to the actions of the subject. Heparinized tubes were used at the time of centrifugation at 3000 rpm for 10 min, and the plasma was stored at -80°C for subsequent chemical analysis. The recovered supernatants were used for all assays. The plasma concentration of progesterone from the sample was analysed. Progesterone concentrations tested before and after the stress test were determined using an enzyme-linked immune sorbent assay (ELISA) (IM1188 Ref, RIA Progesterone) procured from the Czech Republic.
2.3. Exercise Challenge
- The stress test was performed on a cycle ergometer (Model 818, Monark). The ergometer seat, handlebars, and frame were adjusted to the morphology of each subject. The stress test was triangular; the subjects were exposed to a power increment of 25 Watts every three minutes until exhaustion. The inability of the subject to maintain a rate of 70 rev / min indicated fatigue.
2.4. Pulmonary Function Tests
- Flow / volume variables (FVC and FEV) were measured using the Micro Quark Spirometer (Cosmed Ltd., Rome, Italy). Every morning, the spirometer was calibrated using a calibration syringe (Cosmed LLC, Rome, Italy) with a capacity of three liters of atmospheric air. The Micro Quark sets the criteria for the ATS/ERS for measuring individuals of African origin. Indeed, the selection of "African origin" refers to the spirometer equations for African Americans [34]. Mouthpieces and disposable antibacterial filters (Cosmed Ltd., Rome, Italy) were used to avoid cross contamination between subjects. Pulmonary function tests (PFT) were performed under the supervision of a medical technician. After determining the height, body weight, age, sex, and race of the individual, the spirometer unit automatically calculated the value of each respiratory parameter. In the PFT, the individual, while standing with his nose and a turbine between his hands, breathed naturally and quietly through a mouth piece connected to the spirometer. The individual was then instructed to inflate the lungs and then exhale the air as quickly and completely as possible in a continuous way. At the end of the test, the flow-volume curve (FVC + FEV) was calculated according to the acceptance criteria of the ATS / ERS [35]. Exercise-induced bronchospasm was considered positive if the post-exercise FEV decreased by at least 10% from its pre-exercise value.
2.5. Statistical Analysis
- The variables were processed using Stat View 5 software (version 5) (Abacus Concepts Inc. Berkeley, CA, USA). Descriptive statistics were generated for each variable. The normal distribution of the variables, as well as the equivalence of their respective variances, was checked by the Kolmogorov-Smirnov test and the F-test. Comparisons of FVC, FEV and progesterone levels observed before and after the stress test were performed by Wilcoxon tests after the normality of the distribution and homogeneity of variance were not verified. For all statistical analyses, the null hypothesis was rejected at a probability of p < 0.05.
3. Results
- The characteristics of subjects: age; height; weight and Body mass index were presented in table 1. The comparison of FVC and FEV observed before and after stress test where were presented in table 2 and differences were not significant (p > 0.05). In contrast, the progesterone value observed after stress test was significantly (p < 0.01) higher than those observed after test (Table 2).
|
|
4. Discussion
- This study was conducted in non-bronchospasm-sensitive, menstruating female students during the luteal phase to determine whether a significant increase in progesterone induced by a controlled exercise test would induce significant airway constrictions as in asthmatics. Indeed, in asthmatic sportswomen, several studies have highlighted the influence of the phases of the menstrual cycle and sex hormones on their lung function. Consistently, Farha (2009) [19], Kristin et al. (2006) [20] and Pauli et al. (1989) [22] showed a deterioration in exercise-induced bronchospasm during the luteal and follicular phases with more severe asthma symptoms that required the increased use of bronchodilators. Similarly, Wegienka et al. (2012) [36] and Matteis et al. (2014) [37] showed an association between FEV and luteal and follicular phases in asthmatic sportswomen. However, Weinmann et al. (1987) [15] argued that the effect of sex hormones on airway function has not been well elucidated, as numerous studies on the important effect of these hormones on asthmatic women with normal menstrual cycles have shown that changes in sex hormone levels observed during menstrual cycles are not associated with changes in the respiratory airway [15]. However, in the context of this study, the results show that the values of the FVC and the FEV observed at rest compared to those recorded at the end of the stress test do not show a significant difference, despite the significant increase in the concentration of post-exercise progesterone. Our results seem to reinforce those of Weinmann et al. (1987) [15] and Pauli et al. (1989) [22], with the peculiarity that the subjects of our study are not sensitive to the bronchospasm induced by exercise. In this study, a significant elevation in progesterone was observed after exercise. This finding is contrary to the results of Otag et al. (2016) [30], who observed immediately after a stress test, a significant decrease in progesterone in 12 female football players with normal menstrual cycles. In contrast, Bonen et al. (1983) [31], Kraemer et al. (1995) [32] and Otag et al. (2011) [33] found a significantly elevated level of progesterone during the luteal phase of the menstrual cycle that was triggered by physical exercise. Similarly, Shahin Dabhoiwala et al. (2011) [38] found a significant increase in progesterone that did not induce FEV during the secretory phase in 36 adolescent girls with normal menstrual cycles. The results observed by these authors are compatible with ours, but do not explain why a high level of progesterone induced by physical exercise did not influence the respiratory airways in subjects not sensitive to asthma? To our knowledge, this study is the first to address this issue in subjects not sensitive to bronchospasm. The increase in progesterone levels during the luteal phase of the menstrual cycle appears to play an important role in the management of exercise-induced bronchospasm (EIB). Indeed, a study by Stanford et al. (2006) [20] revealed a relationship between elevated progesterone levels and EIB occurrence in amateur athletes. The same authors confirmed that progesterone levels were significantly higher during the luteal phase than during the follicular phase since the FEV and DEP values measured during the luteal phase were significantly lower. This result proves that there is a significant relationship between increased progesterone and decreased FEV. On the one hand, bronchospasm occurs after two sensitizations of mast cells. The first corresponds to the establishment of specific receptors, and the second corresponds to the stimulation of the mast cell membrane to facilitate the penetration of calcium into the cell nucleus, which releases the mediators of inflammation thereafter. On the other hand, Brannan et al. (2003) [39] and Zhao et al. (2001) [25] suggested that the presence of progesterone receptors identified in mast cells plays a role in airway inflammation. Under these conditions, it can be speculated that in athletes sensitive to exercise-induced bronchospasm, progesterone is involved in restoring depressed airways. Indeed, Polly et al. (2009) [40] argued that progesterone has a relaxing effect on smooth muscle and may have a bronchodilator effect in asthmatic individuals. In contrast, even if physical exercise causes a rise in progesterone levels in athletes not sensitive to bronchospasm, the airways may not undergo significant changes since bronchospasm did not occur. This plausible hypothesis may partially explain our results, which would require further investigation to consider the combined effect of progesterone and estrogen and to compare progesterone levels between individuals sensitive and not sensitive to bronchospasm.
5. Conclusions
- Exercise induces an increase in progesterone levels in asthmatic and non-asthmatic patients. While it is clear that progesterone acts as a bronchodilator in asthma-susceptible patients, its use in non-asthmatic-sensitive subjects is not demonstrated in this study. This finding is evidence that sex hormones have a wide variety of effects beyond β2 adrenergic in subjects with exercise-induced asthma and bronchospasm. Further research is needed to investigate this inaction of progesterone in the absence of exercise-induced bronchospasm.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML