-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2019; 9(6): 203-209
doi:10.5923/j.ajmms.20190906.06

Study of B Cell Activating Factor (BAFF) and BAFF-R in Systemic Lupus Erythematosus Patients
Doaa S. D. Zaki1, Nabila M. Ayoub2, Zakaia A. Z. Mohammed2, Hoda H. Faramawy2
1Department of Internal Medicine, Al-Zahraa University Hospital, Al-Azhar University, Cairo, Egypt
2Department of Clinical Pathology, Al-Zahraa University Hospital, Al-Azhar University, Cairo, Egypt
Correspondence to: Doaa S. D. Zaki, Department of Internal Medicine, Al-Zahraa University Hospital, Al-Azhar University, Cairo, Egypt.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background: Elevated levels of B cell–activating factor (BAFF) belonging to the tumor necrosis factor (TNF) family are characteristic of patients with systemic lupus erythematosus (SLE). This cytokine is crucial for the survival, maturation, and differentiation of B cells. Objectives: The aim of this study was to explore the association between BAFF and expression messenger RNA (mRNA) of BAFF receptors (BAFF-R) with clinical activity in SLE patients determined by SLE disease activity index (SLEDAI). Subjects and Methods: Cross sectional observational study was done on a total of 40 females with SLE and 20 age-matched healthy female donors as controls. Serum BAFF was assessed by Quantikine® ELIZA and the expression messenger RNA (mRNA) of BAFF-R was determined by flow cytometry. Results: Serum BAFF levels were significantly increased in SLE patients compared to controls (P < 0.001). The increased circulating BAFF level in SLE patients was positively correlated to SLEDAI score (P< 0.001), CRP, ESR, dsDNA and negatively correlated to complement levels (C3 and C4). However, BAFF-R expression was negatively correlated to SLE activity by SLEDAI, CRP, ESR, dsDNA and positively correlated to complement levels (C3 and C4) in patients with SLE. Conclusion: circulating BAFF levels and BAFF-R expression in SLE patients were correlated to disease activity by SLEDAI and may serve as a peripheral blood biomarker for occurrence of an active disease.
Keywords: Systemic lupus Erythematosus, B cell-activating factor, Disease activity index
Cite this paper: Doaa S. D. Zaki, Nabila M. Ayoub, Zakaia A. Z. Mohammed, Hoda H. Faramawy, Study of B Cell Activating Factor (BAFF) and BAFF-R in Systemic Lupus Erythematosus Patients, American Journal of Medicine and Medical Sciences, Vol. 9 No. 6, 2019, pp. 203-209. doi: 10.5923/j.ajmms.20190906.06.
Article Outline
1. Introduction
- Systemic lupus erythematosus (SLE) is an autoimmune disease that characterized by loss of B-cell tolerance toward nucleic acids and their binding proteins [1]. Activated, autoreactive B cells are responsible for tissue damage by secretion of antibodies and can also drive the loss of tolerance in the T cell compartment [2]. Autoreactive B cells survival and differentiation is largely mediated by a cytokine called B cell–activating factor that belonging to the TNF superfamily (BAFF) [3]. BAFF binds to 3 receptors of the TNF receptor family; the one that essential to the pro-survival effects of BAFF on mature B cells is called BAFF- receptor (BAFF-R). BAFF-R is expressed on all peripheral B cells and some T cells, especially after their activation [4, 5]. Mice lacking BAFF or with defective BAFF-R exhibit failure of survival of the peripheral B cells from immature, transitional stage to the mature, naive stage [6]. The BAFF circulating levels are elevated in the serum of patients with SLE and patients with Sjogren’s syndrome and in the synovial fluid of patients with rheumatoid arthritis [7-9]. Association of autoantibody production with these diseases suggests a potential role of the increased BAFF in the autoimmune disease process. Analysis of BAFF-R in SLE patients revealed that BAFF-R on B cells was occupied to a significantly greater degree in patients with SLE compared with healthy controls [10]. The BAFF/BAFF-R axis has important implications regarding SLE pathogenesis and can be used in treatment of SLE by blockade of this axis. However, the implication of this axis in the disease activity in SLE patients is still controversial. So, we design a cross sectional observational study to correlate the serum BAFF level and the expression messenger RNA (mRNA) of BAFF-R with SLE disease activity which may serve as a peripheral blood biomarker for activity in SLE patients.
2. Subjects and Methods
2.1. Study Subjects
- This was a prospective cross-sectional observational study that included 40 female patients diagnosed with SLE according to American College of Rheumatology criteria [11] and 20 age matched healthy females as controls. The patients were recruited from in-patient department of internal medicine in Al-Zahraa University Hospital, Cairo, Egypt in the period between august 2014 to April 2015. Informed consent was obtained from all study participants in advance. All procedures were performed in accordance with the guidelines in the Declaration of Helsinki and approved by Research Ethics Committee of Faculty of Medicine for Girls, Al-Azhar University. Exclusion criteria includes other autoimmune diseases, chronic liver diseases, type-1 diabetes and malignancy. All patients were subjected to a standardized questionnaire which specially emphasizes duration of the disease and SLE manifestations. Physical examination was done to all patients and disease activity was assessed by the SLE Disease Activity Index (SLEDAI) on the day of blood testing [12]. SLEDAI is an easy valid and reliable index for assessment of activity by revising twenty-four features that are attributed to lupus with a weighted score given to any one that is present [13]. Mild disease activity score < 10, moderate disease activity score from 10-18 and sever disease activity score > 18 [12].
2.2. Serum BAFF Assay
- Serum BAFF was assessed by enzyme quantitative immunoassay using Quantikine® ELIZA (human BAFF/BLyS/TNFSF13B Immunoassay-catalog number DBLYS0B Catalog Number) on ELISA system (Reader A3 1851 & Washer 909) from das (Italy).
2.3. BAFF-R Expression Analysis
- Flow cytometric analysis of BAFF-R was done by Becton Dickinson Facs caliber flowcytometry with a standard 4-coloor filter configuration using R and D system kits. Figures 1, 2 and 3 representing mild, moderate and high co-expression of BAFF-R & CD19 respectively.
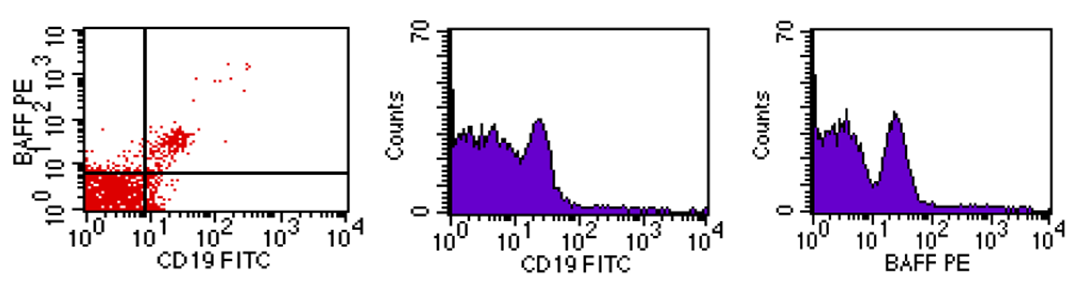 | Figure 1. Mild co-expression of BAFF-R & CD19 |
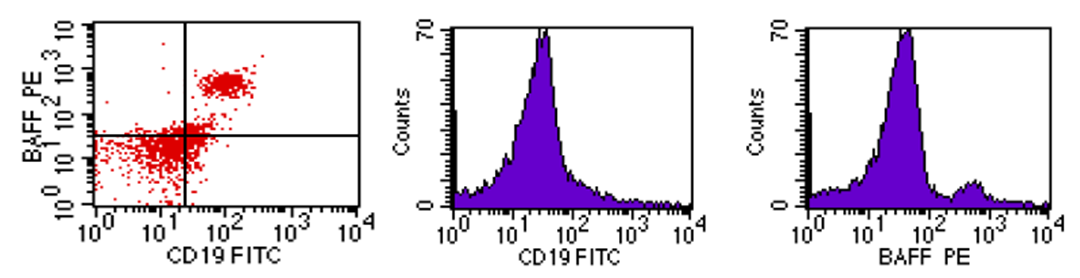 | Figure 2. Moderate co-expression of BAFF-R & CD19 |
 | Figure 3. High co-expression of BAFF-R & CD19 |
2.4. Statistical Analysis
- Data were collected, revised, coded and entered to the Statistical Package for Social Science (IBM SPSS) version 20. The qualitative data were presented as number and percentages. The parametric quantitative data were presented as mean ± standard deviations and ranges while the non-parametric data were presented as median with interquartile range (IQR). The comparison between two groups with qualitative data were done by using Chi-square test. The comparison between two independent groups with quantitative data and parametric distribution was done by using Independent t-test and non-parametric distribution was done by using Mann-Whitney test. The comparison between more than two independent groups with quantitative data and parametric distribution was done by using One Way Analysis of Variance (ANOVA) and non-parametric distribution was done by using Kruskall-Wallis test. Spearman correlation coefficients were used to assess the correlation between two quantitative parameters in the same group. Receiver operating characteristic curve (ROC) was used to assess the best cut off point with its sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and area under curve (AUC). The confidence interval was set to 95% and the margin of error accepted was set to 5%. So, the p-value was considered non-significant if P > 0.05, significant if P < 0.05 and highly significant when P < 0.001.
3. Results
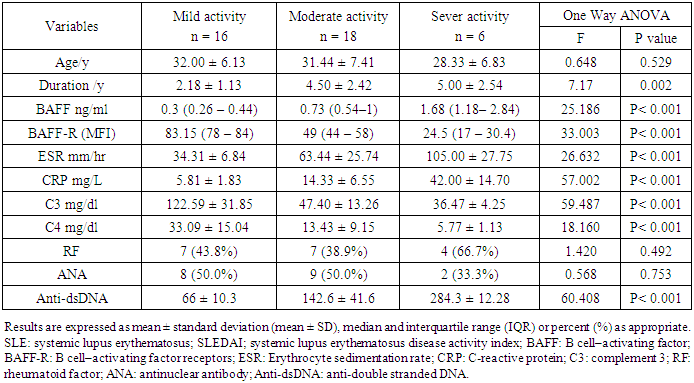
- In the present study, 40 SLE patients (all were females with mean age of 31.2 ± 6.8 years ranged between 20 and 47 years) and 20 sex and age - matched 20 healthy controls (all were females with mean age 31.5 ± 5.0 years ranged between 23 – 40 years), P > 0.05, were included. SLE patient’s characteristics were represented by table 1; the mean disease duration in SLE patients were 4.4 ± 2.5 years. Musculoskeletal and dermatological manifestations were the most common clinical presentation in SLE that present in 100 % of patients at the time of sample collection, followed by constitutional and hematological manifestation (67%), GIT (65%), cardiac (52%) and renal (50%) manifestations. Other manifestations were less common in the study patients. Rheumatoid factor (RF) was positive in 55% and ANA in 52.5% of SLE patients.
|
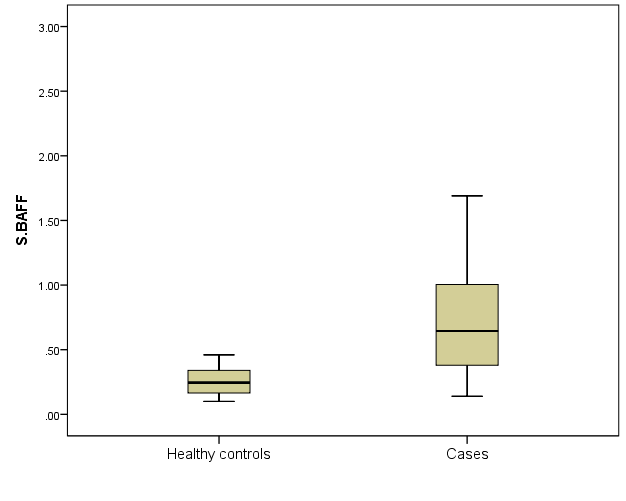 | Figure 4. Serum BAFF level in SLE patients and healthy controls |
 | Figure 5. Sensitivity and specificity of BAFF in SLE patients |
|
4. Discussion
- The crucial B cell survival factor BAFF has an important role in autoimmunity particularly in SLE pathogenesis. It maintains B cell homeostasis by acting as survival and activation factor for B cells from the transitional stage of development onwards [14]. This effect is mediated mainly by BAFF-R which is the main receptor transmitting the BAFF signal to B cells [15]. Several studies were reported increased circulating level of BAFF in sera of SLE patients [7, 16-19]. BAFF/BAFF-R axis appears to span nearly all stages of B-lineage differentiation. Thus, the dysregulation of BAFF/BAFF-R system may contribute to induction and development of autoimmune diseases; hence, it may be considered as an important therapeutic target [20]. Despite the insights on abnormalities of blood B cell subsets in SLE, a peripheral blood biomarker with useful clinical information about the occurrence of an active disease period hasn’t yet been achieved and attempts to correlate disease activity with the circulating BAFF and BAFF -R have been largely controversies.In the present study, we found significantly elevated serum BAFF level in SLE patients compared to sex and age-matched healthy controls (0.65 (0.38 – 1) versus 0.25 (0.17 – 0.34) respectively) P < 0.001. The increased circulating BAFF was positively correlated to SLEDAI score (P< 0.001) and was more pronounced in the sever active group than in the less active groups (P< 0.001). Importantly, while levels of serum BAFF correlated positively with disease activity in our patients, levels of BAFF-R expression were correlated negatively with SLEDAI score (P< 0.001) and was gradually decreased with activity showing lowest expression in the sever active group (P< 0.001). Decreased detectable level of BAFF-R in active SLE patients can be explained by occupation of more BAFF-R by high BAFF levels with activity. Interaction of BAFF with BAFF-R stimulates the classic nuclear factor-κB pathway, enhancing B cell survival, growth, and metabolic fitness [15].Earlier study on SLE patients was reported that BAFF mRNA and soluble BAFF levels were highest in the active SLE group, followed by the stable SLE group, and controls, and the percentage BAFF-R on B lymphocytes was downregulated in the active SLE group compared with the stable SLE group and controls [21]. However, the relation with other laboratory markers of activity have not been correlated. Circulating BAFF levels in SLE patients in the present study was correlated positively with other laboratory markers of activity including CRP, ESR, dsDNA and negatively with complement levels (C3 and C4). In a previous case control study, the association between BAFF level and acute-phase responses in SLE patients was not dependent on BAFF genotype or expression and was due to disease activity that indicating BAFF production at sites of inflammation [24]. However, other studies failed to prove this correlation [25, 26]. This controversy may be attributed to differences in disease activity scoring system used or different BAFF isoforms [27].BAFF-IgG complexes in sera of SLE patients was also strongly correlate with disease activity, suggesting a pathogenic role in SLE [22, 23].As regard to BAFF-R expression, it was lowest in SLE patients with severe activity as compared to less active groups and negatively correlated to SLE activity by SLEDAI (P< 0.001). Signalling through BAFF-R, that activates the classical factor-κB pathway, upregulating anti-apoptotic proteins [28]. It can also signal through extracellular signal regulated kinase (Mek/Erk) and through molecular target of rapamycin (Akt/mTOR) that mediate B-cell survival by repressing the proapoptotic protein Bim [29]. Earlier study of BAFF-R occupancy on blood B cells reported consistent occupation of this receptor in SLE patients. The extent of reduction of available receptors was correlated with disease activity [10]. However, they did not correlate BAFF-R expression with other laboratory markers of activity. In the present study, BAFF-R expression was negatively correlated to acute phase reactant and may contribute to disease mechanisms that may be targeted in SLE therapy.Another study in Chinese SLE patients reported negative expression rate of BAFF-R on CD19+ B cells with disease activity by SLEDAI [30]. The increased occupancy of BAFF-R in SLE implies that in treatment strategies based on targeting of BAFF, the binding of BAFF to BAFF-R will have to be overcome to successfully reduce the biologic activity of BAFF and thereby modulate B cell activation in this disease.
5. Conclusions
- Our study suggests that circulating BAFF level and BAFF-R expression in SLE patients were correlated to disease activity and may serve as a peripheral blood biomarker for occurrence of an active disease.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML