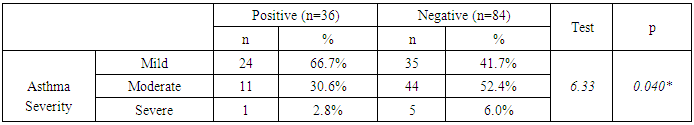-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2019; 9(4): 151-156
doi:10.5923/j.ajmms.20190904.05

Study the Correlation of Helicobacter Pylori Infection with Bronchial Asthma and Its Relation to Asthma Severity
Atef Elrifai1, Hany Awadallah2, Ahmed Salama Al Adl3, Mohamed M. Aldesoky4
1Chest Disease Department Al-Azhar University Faculty of Medicine Damietta, Cairo, Egypt
2Tropical Medicine Department Al-Azhar University Faculty of Medicine Damietta, Cairo, Egypt
3Internal Medicine Department Al-Azhar University Faculty of Medicine Damietta, Cairo, Egypt
4Microbiology Department, Al-Azhar University Faculty of Medicine Damietta, Egypt, Cairo, Egypt
Correspondence to: Ahmed Salama Al Adl, Internal Medicine Department Al-Azhar University Faculty of Medicine Damietta, Cairo, Egypt.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background: Helicobacter pylori (HP) infection was linked with allergic diseases and asthma. However some studies reported contradictory results. Aim of the study: Evaluation the Role of Helicobacter pylori infection in asthmatic patients and its Relation to Asthma Severity. Patients and methods: the current work included 120 patients with Bronchial Asthma and 60 healthy persons as control. All were submitted to history taking, clinical examinations, laboratory and radiological investigations. HP was diagnosed by antigen detection in stool. Results: HP was reported in 36 patients with bronchial asthma (30.0%) and 25 healthy subjects (41.7%) with non-significant difference between both groups. asthmatic patients with positive HP infection revealed good pulmonary function tests, lower IgE antibodies, and significantly higher antibodies titer against HP. In addition, H-pylori infection was significantly prevalent with mild asthma (66.7%), then moderate (30.6%) and finally severe asthma (2.8%). The Serum level of HP antibody titer was inversely correlated with FEV1/FVC and IgE. Positively correlated with pre FEV1. Thus, we could say that, asthma severity is inversely correlated with H-pylori infection. Conclusion: Asthma severity was negatively correlated with HP infection, patients with Bronchial Asthma and HP infection revealed good pulmonary function tests and lower IgE antibodies that favor the protective role of HP infection in those patients.
Keywords: Bronchial asthma, Helicobacter pylori, Atopy, Allergy
Cite this paper: Atef Elrifai, Hany Awadallah, Ahmed Salama Al Adl, Mohamed M. Aldesoky, Study the Correlation of Helicobacter Pylori Infection with Bronchial Asthma and Its Relation to Asthma Severity, American Journal of Medicine and Medical Sciences, Vol. 9 No. 4, 2019, pp. 151-156. doi: 10.5923/j.ajmms.20190904.05.
Article Outline
1. Introduction
- Bronchial asthma is a national wide disorder, and more than 300 million peoples were affected all over the world. The disease prevalence in developed countries has risen in a dramatic way, that it has reached an epidemic proportion. The definite cause leading to such increase remains largely unrecognized [1]. However, proposed risk factors include food-borne and oro-fecal infestations [2], changes in smoking habit [3], presence of pet animals [4], type of houses [5], family size, income and education [6], and different particulate matters in diesel exhaust [7]. In addition, helicobacter pylori infestation was blamed to be linked with asthma [8]. As a gastric pathogen, helicobacter pylori are responsible for the occurrence of peptic ulcers, gastric adenocarcinoma, and primary gastric lymphoma [9]. The incidence of H. pylori infestation (HPI) varies greatly between developed and developing countries (10%–20% in developed, and 80%–90% in developing countries) [10]. The HP infection may attain in childhood [11]. H. pylori induce chronic inflammatory response that in turn induces numerous extra-gastric conditions, such as coronary artery disease, metabolic syndrome [12], and dementia [13]. On the opposite side, it was reported that, HP infection has been linked with reduction of allergic diseases [14] and childhood-onset asthma [15]. However, there are others studies that have failed to document the negative association between bronchial asthma and HPI [16].
2. Aim of the Study
- Evaluation the role of helicobacter pylori infection in asthmatic patients and its relation to asthma severity.
3. Patients and Methods
3.1. Study Design
- The current study was conducted at Al-Azhar University Hospital (New Damietta), from November 2015 to November 2017. It included 120 patients with bronchial asthma. Another 60 subjects without bronchial asthma age and sex matched were included as a control group. Asthma was diagnosed if the patient had repeated attacks of chest wheezing, chest tightness, dyspnea, FEV1/FVC < 70% and 12% increase in FEV1 post- bronchodilator; and patients then classified according to asthma severity into mild, moderate and severe [17].
3.2. Ethical Aspects
- The study protocol was accepted by the local ethics and research committee, and consent was signed by the guardian.
3.3. Exclusion Criteria
- The patient was excluded if he/she had cardiac disease, hypertension, lung disorders other than bronchial asthma, or neuromuscular disease.
3.4. Inclusion Criteria
- All asthmatic patients presented to our hospital. The bronchial asthma was diagnosed clinically if the patient had repeated attacks of chest wheezing, chest tightness, dyspnea, and by Spirometry FEV1/FVC < 70% and 12% increase in FEV1 post- bronchodilator [17].
3.5. Study Protocol
- All patients were underwent history taking, clinical examination, chest x-ray, Spirometry, detection of helicobacter pylori Antigen in stool, serum helicobacter pylori IgG antibody titer and determination of plasma IgE. Serum H. pylori Antibody Test. About 5 ml of venous blood were obtained from antecubital vein after 12 hours of fasting. The samples were centrifuged and plasma was separated, and used for estimation of serum anti-H. Pylori IgG antibodies and their values were determined using an ELISA kit ((DiaPro Co. Ltd., Milan, Italy). The values of serum anti-H. Pylori IgG antibody assay were presented as negative (< 10), mild positive (10-100), moderate positive (101-200) and strongly positive (> 200).Patients with bronchial asthma were classified into two subgroups according to results of helicobacter pylori Antigen in stool. The first included patients with positive H-pylori and the second included patients negative for H-pylori infection [18]. Assay of plasma IgE. It was done by Human IgE ELISA Quantification Kit as described by the producer’s directions. Optical densities were determined at 450 nm with a wavelength of 595 nm as a reference. Levels of IgE were calculated from a standard curve. Detection of HP antigen in stool: specimens were obtained (after stoppage of any medications which interfere with the test) and examined using the stool antigen test kits (Rapid Immunoassay method, GA GENERIC ASSAYS GmbH, Germany) and values were reported as positive or negative.
3.6. Statistical Analysis
- Data were analyzed by statistical package for social science (SPSS), version 20 (IBM®SPSS®, Chicago, Illinois, USA). The quantitative data were stated as mean and standard deviation (SD), while qualitative variables were conveyed as frequency and percent. The groups were compared by independent samples (t) and Chi square test for quantitative and qualitative data respectively. P value ≤ 0.05 was considered significant.
4. Results
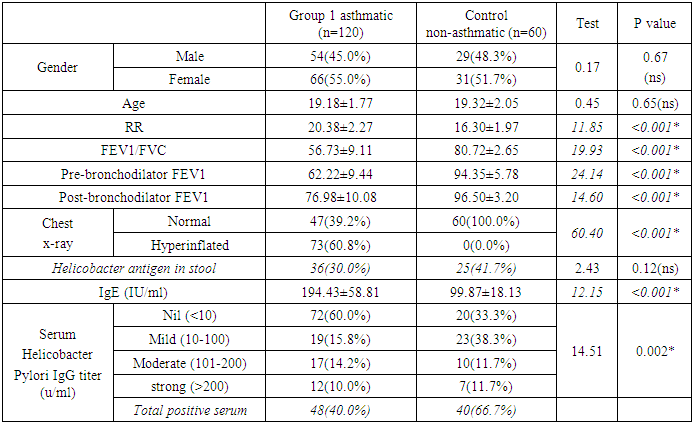
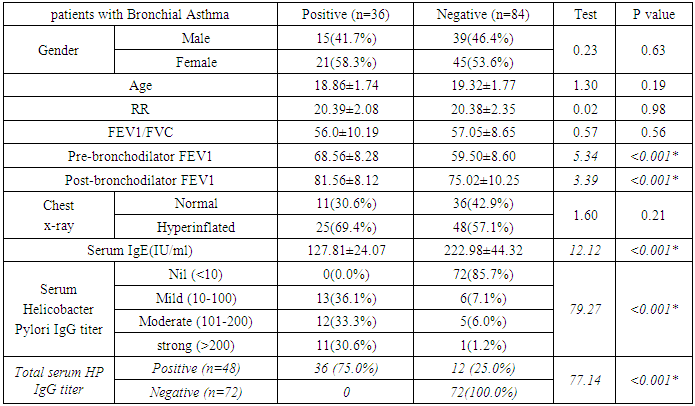
- HP was reported in 36 patients with bronchial asthma (30.0%) and 25 healthy subjects (41.7%) with non-significant difference between groups. There was statistically insignificant difference between study and control groups as regard to patient age (18.18±1.77 vs 19.32±2.05 respectively) or patient gender (males constituted 45.0% and 48.3% of study and control groups respectively). The study (asthmatic) group had significantly increased respiratory rate, significantly lower FEV1/FVC and significant reduction of pre- and post-bronchodilator FEV1. The chest X-ray was normal in all control subjects and in 39.2% of asthmatic patients. There was statistically significant increase of serum IgE in study when compared to control group (194.43±58.81 vs 99.87±18.13 respectively). On the opposite side, there was significant decrease of positive H-pylori infection in serum in study when compared to control group (40% vs 66.7 respectively). (table 1). When comparing H-pylori positive and negative patients, there was insignificant difference between both subgroups as regard to patient gender, age, RR, FEV1/FVC, chest x-ray. However, positive subgroup had significantly increased pre- and post-bronchodilator FEV1, significantly reduced IgE and significant increase of positive H-pylori by serum antigen. The sensitivity of serum H-pylori antigen detection when compared to stool antigen test was 75% and specificity was 100.0% (table 2). H-pylori infection was significantly prevalent with mild asthma (66.7%), then moderate (30.6%) and finally severe asthma (2.8%) (Table 3). Serum level of HP antibody titer was inversely correlated with FEV1/FVC and IgE. And positively correlated with pre FEV1 (figure 1, 2, 3) Thus, we could say that, asthma severity is inversely correlated with H-pylori infestation.
|
|
|
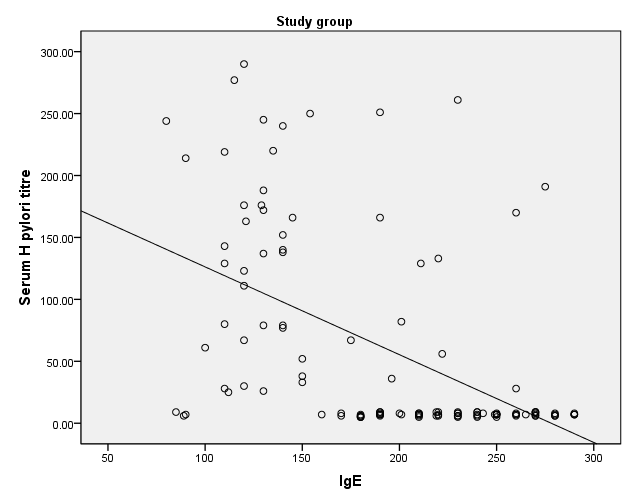 | Figure 1. Correlation between Serum level of HP antibody titer with IgE |
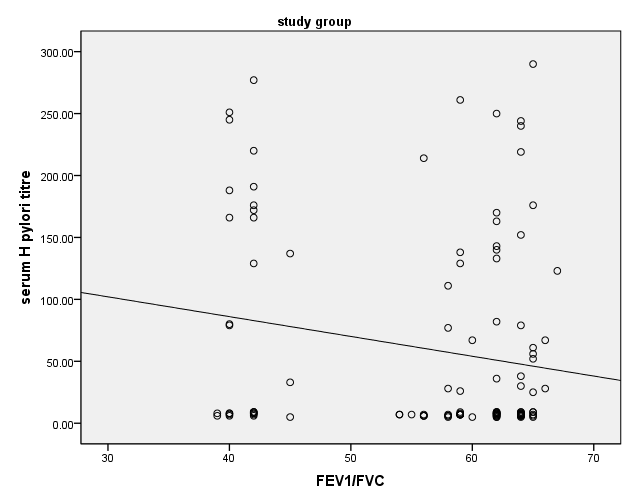 | Figure 2. Correlation between Serum level of HP antibody titer with FEV1/FVC |
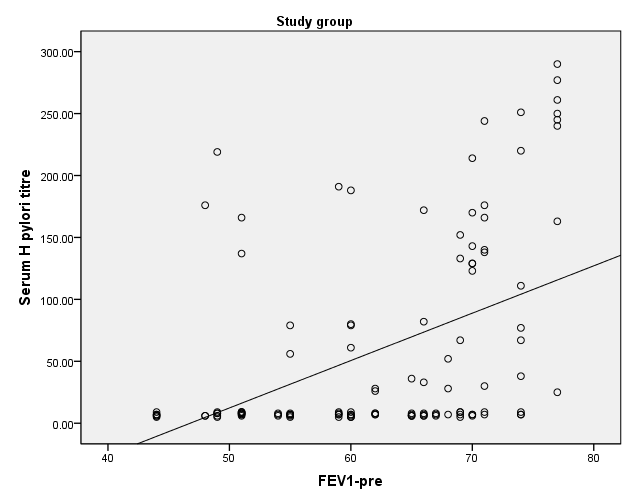 | Figure 3. Correlation between Serum level of HP antibody titer with pre FEV1 |
5. Discussion
- There are an association between allergic diseases and H. pylori infection, it was suggested that the two diseases might be related. However, the topic is still controversial. Thus, the present work was designed to detect the probable link between bronchial asthma (as an allergic disease) and H. Pylori infection. 120 patients with bronchial asthma and 60 age and sex matched healthy persons as controls were included. H. pylori infection was designed by antigen detection in stools. Results of the present work revealed that, H. pylori infection was noticed in (30.0%) of asthmatic when compared to control (41.7%) and the difference was statistically non-significant. In addition, in asthmatic patients, the H. Pylori infection was significantly increased in mild conditions (66.7%), and then decreased progressively in moderate and severe condition (30.6% and 2.8% respectively). Furthermore, respiratory functions were markedly hastened in positive when compared to negative asthmatic patients, with significant decrease of IgE in asthmatic patients with positive H. pylori infection. Results of the present work revealed also that Serum level of HP antibody titer was inversely correlated with FEV1/FVC and IgE. Positively correlated with pre FEV1. These data collectively indicated that, there is a relation between asthma and H. Pylori infection that inversely correlated with asthma severity. These data are in agreement with Zhou et al. [19] in their meta-analysis; found that H. pylori infection was meaningfully infrequent in patients with asthma than among controls. Another meta-analysis revealed a significant inverse association between H. pylori and asthma [20]. In addition, results are in accordance with Chen and Blaser [21] who conducted a large trial that included 7,412 adult patients and showed that H. pylori infection was inversely correlated with asthma and wheezing. The same authors in a previous work [22] showed that antibody to the H. pylori cytotoxin-associated gene product (CagA), an indicator of severe inflammatory type of H. pylori infection, was inversely correlated with asthma. Thus, and according to these studies H. pylori infection was proposed to play a protecting role against asthma and allergy. To explain protecting role of H. pylori infection in asthmatic patients, it was reported that, early exposure to environmental and microbial factors can build up the developing immune system and provide protection against consequent immune-mediated disorders (hygiene hypothesis) [23]. Thus, commensal microflora or probiotic bacteria with different antigens may play a role in reduction of the individual risk of allergy [24]. On the opposite side, there were a number of studies that deny the association between H. pylori infection and asthma. For example Radon et al. [25] reported that, IgE antibodies were not linked to H. pylori antibodies in Germans. In addition, Annagur et al. [26] reported that, the skin test for allergy failed to distinguish between those with or without asthma. Furthermore Tsang et al. [27] found a high H. pylori in asthmatic, although the difference was statistically non-significant. Also, Lee et al. [28] reported that, patients with past H. pylori had a significant high prevalence of allergy and den Hollander et al. [29] found a high incidence of recent asthma in H. pylori-positive when compared to H. pylori- negative infection. The possible explanation for these contradictory results may attributed to many factors: first the different study designs, for example we included only asthmatic patients, while many of previous studies included different allergic diseases such as hay fever, atopy in addition to asthmatic patients. Second, the multifactorial nature of the asthma, where interaction between environmental and host genes play a significant role in triggering of asthma [30].
6. Conclusions
- Asthma severity was negatively correlated with HP infection, patients with Bronchial Asthma and HP infection revealed good pulmonary function tests and lower IgE antibodies that favor the protective role of HP infection in those patients.
Abbreviations
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML