Ajobiewe H. F.1, Ajobiewe J. O.1, 2, Mbagwu T. T.1, Ale T.2, Maria E.1
1Biological Sciences Department Bingham University Karu, Nasarawa State, Nigeria
2Microbiology Department National Hospital Abuja, FCT, Nigeria
Correspondence to: Ajobiewe J. O., Biological Sciences Department Bingham University Karu, Nasarawa State, Nigeria.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
This study’s principal aim was to critically appraise onchocerca microfilariae density in various breeds, ages, and sex of cattle. In the method applied according to Cheesbrough, (2001), a total of 838 randomly sampled microfilariae were recorded from the various breeds of cattle; of these 414, 232 and 182 were recorded from the umbilical region, nuchal ligament and the neck respectively. The result showed that the density of microfilariae was highest in August, with 245, followed by March, with 215. Microfilarial density of 2.03 per skin snip was obtained. Reduction in Onchocerca volvulus skin microfilaria density after treatment with ivermectin showed wide range in host variations. Three species of Bovine Oncochocercaciasis encountered during this study were Onchocerca gutturosa, Onchocerca duckei, and Onchocerca giboni which showed significant variations in various anatomical sites (P < 0.05). Of this number, 146 (48.66%) were infected with the various species of Onchocerca, accordingly: Onchocerca gutturosa 92(63.0%), Onchocerca duckei 51(34.93%), and Onchocerca giboni 3 (2.05%). It was also observed that the prevalence of Bovine Onchocerciasis were higher in animal aged (5-6years) followed by those between (3-4 years) and lastly those aged 7years and above (P<0.05). Significant difference also existed between breed of cattle and infection. Bovine Onchocerciasis displayed seasonal variations in wet season (50.54%) being higher than dry season (47.83%).
Keywords:
Bovine Onchocerciasis, Season, Onchocerca gibsoni
Cite this paper: Ajobiewe H. F., Ajobiewe J. O., Mbagwu T. T., Ale T., Maria E., Critical Appraisal of Onchocerca Microfilariae Density in Various Breeds, Ages, and Sex of Cattle in Karu Local Government Nassarawa State Nigeria, American Journal of Medicine and Medical Sciences, Vol. 9 No. 4, 2019, pp. 131-142. doi: 10.5923/j.ajmms.20190904.01.
1. Study Background
Various species of Onchocerca especially Onchocerca gutturosa in the ligamentum nuchae, gastro splenic ligament e.t.c can be detected only on postmortem examination, although nodules of other species may be palpated in the subcutaneous tissue (Naik, 2002). There are specific locations or predilection sites for the skin dwelling microfilariae species within the Bovine host, though not much studies has been conducted in this area, previous works have been limited to areas along the cervical spine, Ears brachium and umbilicus Ventatarafnan and Kershaw, (2006); Kolstrup, (1993); Eberhand (1999). Studies by Copeman (1989) have shown that when the umbilical and cervical habour microfilariae, they are always present in highest concentration and the reason given was that microfilariae localize in the umbilical region regardless of the location of the adult worm so as to ensure transmission by S. ornatum (Eichler and Nelson 1997; Harty et al., 1989). It was also observed that microfilariae can be obtained from the umbilical cord, nuchal and neck region as stated earlier. This information is to a corresponding general theory of epidemiology of the disease, and thirty, to determine in detail the local conditions which favors or control the dissemination of this infection in the given area. In general, epidemiological evidence therefore consists of interrelated facts from which a conclusion or series of conclusions may be drawn.Epidemiological factors affecting the vector Schistosoma ornatum alighting and biting activities includes wind; few flies come for blood-meal if there’s the slightest of breeze. David’s, (1991) also noted that a wind speed of 5.m.p.h usually completely inhibited activity. It completely stops if precipitation consisting of more than a very slight shower occurred. In general the greatest activity appeared to take place in warm, calm days particularly if the humidity was high (Shasri, 1998). David’s (1991) observed that the strength of illumination of the surface of cattle by sunlight is a major factor in controlling the alighting activity of Simulium ornatum. That observed that the flies settled mainly on the ventral region of the animal rather than elsewhere but when sunlight reflected unto the umbilicus during period of heavy biting, this resulted in a rapid and marked decrease in the number of flies feeding, as feeding flies were not distributed but fresh flies did not start feeding until the mirror was removed. On one occasion light was reflected unto the umbilicus continually for twenty minutes, during which only few flies settled and fed on a part shaded by a fold on the skin but when the mirror was removed, flies began to arrive and feed on the umbilicus within a few seconds. Ventatarafnan and Kershaw (2006) showed in their study that the distribution of microfilariae of Onchocerca species in the cattle varied in different areas of the skin according to the site of the adult worm, when the worm is present only in the neck, microfilariae were found in the ear skin and near-by but when splenic worms are present many of the ear skin snip were negative. The distribution of microfilariae in the umbilical region of the skin has an effect on the transmission of Onchocerca gutturosa and the distribution of the adult worms of Onchocerca gutturosa and its larvae has been described (Eichler and Nelson 1997). Almost all the microfilariae migrate to the umbilicus and so have maximum opportunity of being ingested by the vector. Not only do the microfilariae concentrate on the umbilical skin, but majority are found at its Centre, where Simulium ornatum has the greatest opportunity of penetrating the hairs and taking a blood meal; a striking example of the adaptation of a parasite to the habit of its intermediate host (Adeleke, et al 1998). For Bovine Onchocerca to be transmitted there must be a source of Simulium ornatum and or cullicoide species of fly whose breeding places are in the forest or savannah regions. Along the courses of free flowing, shallow and well oxygenated rivers and streams with flint gravel bed in the case of Simulium, or in swampy areas along the banks of rivers, pools, in muds and sand soaked by sea water and around decaying trees and vegetation including rotten banana tree stumps in the case of Cullicoides, thus if all these factors are eradicated epidemiology is affected. Factors affecting transmission includes the cattle-fly contact.The intensity of this contact depends mainly on: The density of the vector and the cattle population. The bionomics of the vector, its host preference, dispersal, migration and daily biting activity: these may all vary at different times of the year depending on the seasonal changes and climatic conditions (Brinkman 1980). Cattle factors such as location of cattle, daily or seasonal activities and migration in relation to breeding sites and movements of inter-mediate host are necessary. Factors influencing the intake and development of the parasite, the major factor being determined by the proportion of vectors becoming infected with Bovine Onchocerca species as the prevalence and intensity of microfilariae in the cow population.Treatment of Bovine Onchocerciasis in many developed countries has been possible by the use of DEC and ivermectin. A number of studies have been conducted Coulard, et al., (1983); Awadzi et al., (2005); Green et al., (1995); Aziz et al., (1992) and more are still going on, all geared towards incorporating ivermectin into the primary health care of many countries. It is worthy to observe that results from these studies in the treatment of Onchocerciasis have been encouraging as ivermectin in most cases has compared favorably with DEC which is currently a reference drug. Ivermectin has also been reported to show a number of advantages over DEC with particular reference to adverse effects or reactions in Onchocerciasis victims. This may have control and epidemiological implications especially with regard to mass chemotherapy (Townson et al., 1996). Onchocerciasis and its black fly vectors are rather wide-spread in Nigeria. In particular about 50, 000 people are known to suffer from the disease. Efforts have recently been directed to establishing various essential base line information on the prevalence of Onchocerciaisis in various parts of plateau state (Onwuliri and Eno; 1995) as well as documenting the relative abundance and circadian activity of the black fly species that transmits the disease (Obed, G. 2017; Roberts and Irving Bell, 1985). However, data on the aspect of Bovine Filariasis in part of Northern Nigeria is very scanty (Anosike, 1992). The various species of Bovine Onchocerca exhibit almost a similar pattern of reproduction which is cyclical in introduction into host cell, though length of cycle varies with temperature and may be prolonged several weeks beyond the approximate given time, the time of molting and rates of development correspondingly vary. Onchocerca gibsoni has been known to be haboured by midges, cullicoides species which serve as intermediate host. The life cycle of Onchocerca gutturosa resembles that of Onchocerca cervicalis in horse but rather than development to take place in cullicoides, it occurs in the blackfly. Fly Schistosoma ornatum (intermediate host). Onchocerca gutturosa can also develop in Schistosoma erythrocephallum but not in cullicoides. Attempts to get it develop in Stomoxys calcitrans and species of the genus Musca failed. Further works of Steward (1998) conducted near Cambridge in England on Onchocerca gutturosa showed that microfilaria ingested by Simulium develop into infective larva within three weeks. His observations on other cattle- biting flies and midges indicated that Schistosoma ornatum was probably the only vector in the area. Subsequently, Gnedian, (1996) also described the parasite’s development in the same Simulium species in Russia and Australia, but a research conducted in Kyusu, Southern Japan (1975-1977) on Onchocerca of cattle suggested that Schistosoma ornatum is also a vector of Onchocerca gutturosa in cattle.
2. Method
In the sample collection, biopsies of about 2mm in diameters were taken from the shaved sites (nuchal crest, umbilicus and neck) of fleshly slaughtered cattle for onward transmission to the laboratory for examination. The skin biopsies were each preserved in 5ml volume of physiological saline in universal bottles at the abattoir for gradual emergence of filarial parasites before proceeding to the laboratory. Prior to sample collection, the slaughtered animals were examined to determine the sex, breed and age. Age determination was carried out by counting the number of permanent incisors in the animal. Sterile, sharp surgical blades, needles and hand gloves were employed during the exercise, with adequate precaution to minimize accident. Wet mounted and incubated for three hours and thereafter: A 5 ml sample was incubated for three hours and thereafter transferred into test tube and centrifuged at 3.00pm for 5 minutes. With the aid of clean pastures pipettes, Deposits were transferred to clean slides, covered with cover slip and viewed for presence of microfilaria worms from the various biopsies. Isolates were studied for differentiation into species using unique characteristics features shown in the table below. Non-positive samples were subjected to further incubation with addition of 5mg of Streptomycin and procaine penicillin antibiotics overnight. This antibiotic addition arrested invasion of the sample by bacteria. The re-incubated samples were all re-examined microscopically for quality assurance. Giemsa and Fields staining methods were adopted as described by Monica Cheesbrough, (2000).
3. Hypothesis
H0; No Significant difference existed in the occurrence of the various species of Onchocerca infecting cattle and the microfilariae density as appraised by their breeds, ages and sex.Ha; Significant difference existed in the occurrence of the various species of Onchocerca infecting cattle and the microfilariae density as appraised by their breeds, ages and sex.
4. Result
Table 1. Breed-related prevalence of infection during the study period
 |
| |
|
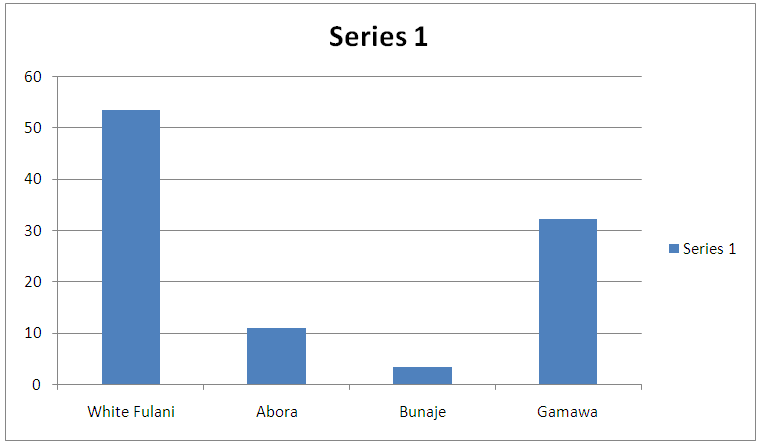 | Figure 1. Breed-related prevalence of infection during the study period |
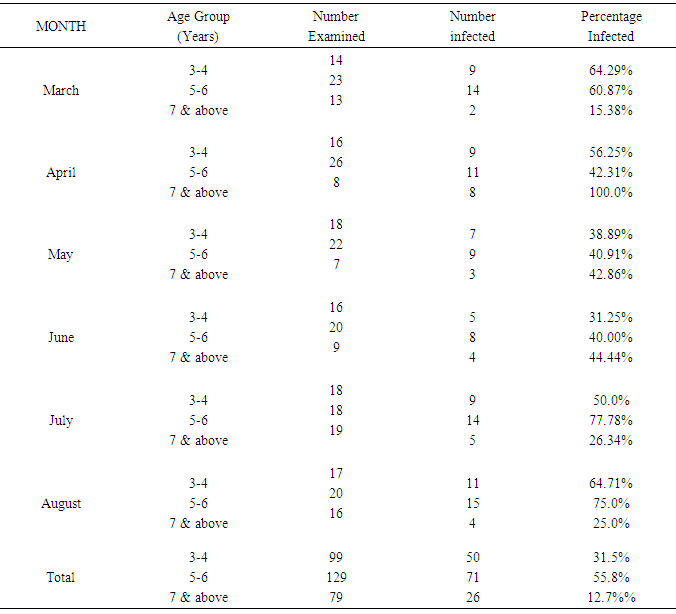
Table 2. Age related prevalence of Bovine Onchocerciasis during the study period
 |
| |
|
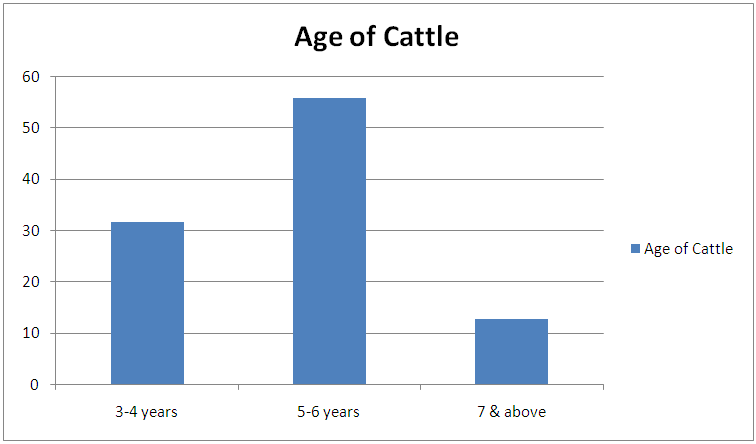 | Figure 2. Age related prevalence of Bovine Onchocerciasis during the study period |
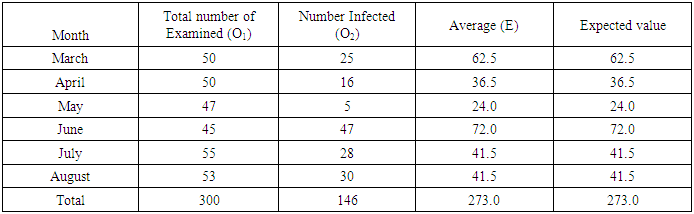
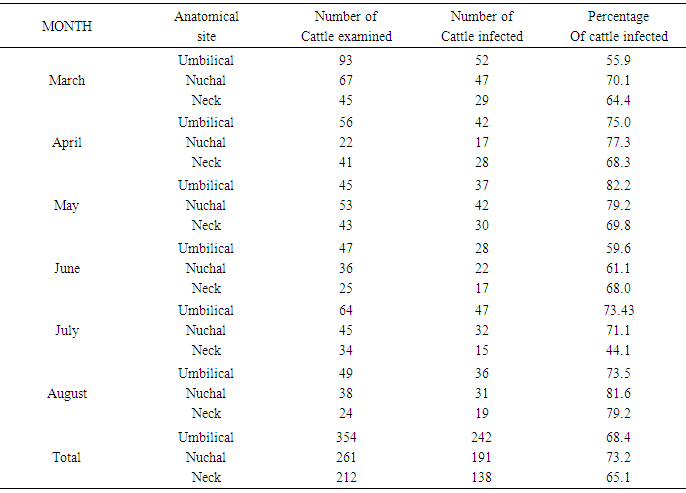
ANATOMICAL SITE AND SPECIES RELATED PREVALENCEFrom the umbilicus, a total of 354 snips taken from the 300 cattle were examined 92 (63.0%) was positive for Onchocerca gutturosa, and from the nuchal crest 261 Snips were collected with (3.5%) having Onchocerca gutturosa, Onchocerca dukei, with one having Onchocerca gibsoni. 57 (34.93%). Positive results, was recorded from the 212 skin snips from the neck, representing (26.6%), and all were positive for Onchocerca gutturosa and Onchocerca. Gibsoni.Microfilariae DensityA total of 828 microfilariae was recorded from the 146 positive cattle; of these 414, 232 and 182 microfilariae were recorded from the umbilical region, nuchal ligament and the neck respectively. The density of microfilariae was highest in August with 245, followed by March with 215. Microfilarial density of 2.03 per skin snip was obtained. Reduction in Onchocerca volvulus skin microfilaria density after treatment with ivermectin shows wide range between host variations. Data from two separate studies conducted in Cameron Onchorcerciasis patients’ treatment for the first time with invernectin were analyzed to identify host factors associated with microfilaria density at different time points after treatment. Onchocerca nodules and microfilaria densities on D0 (pre-treatment), D15, D80 and D180 were available. In the other site, (Vilna Valley) analyses were conducted on 965 individuals of both sexes, aged 5 years and above. In this data, set available, information included age, gender, extra dose of invermectin received, Onchocerciasis endemicity level in the village of residence and microfilaria densities on D0, and D180, negative binomial regression of microfilaria density at different intervals, post-treatment were filtered using maximum likelihood, with the available independent variables. A chi square (λ2) to show the infection rate relative to monthly period of the study
 |
| |
|
 λ2 Tabulated = 25.00Calculated λ2 is greater than tabulated λ2 so there was significant difference in the prevalence of infection at the course of this research work. (P<0.05)
λ2 Tabulated = 25.00Calculated λ2 is greater than tabulated λ2 so there was significant difference in the prevalence of infection at the course of this research work. (P<0.05)Table 3. Microfilariae density encountered during the study period
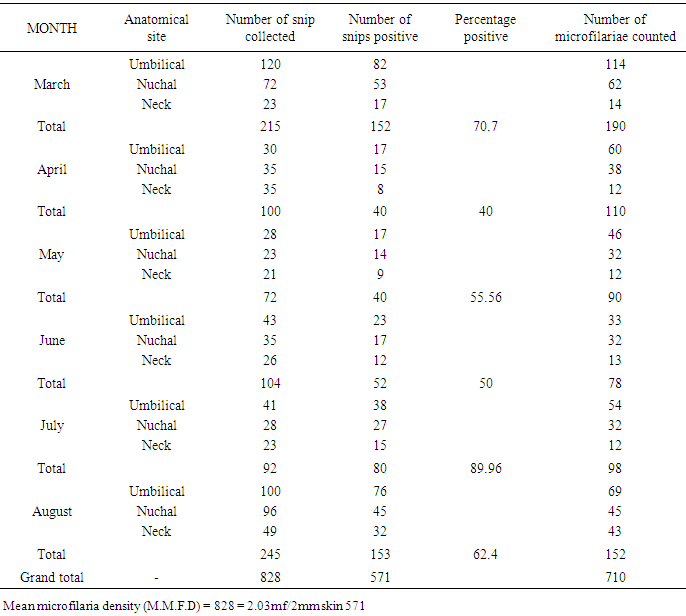 |
| |
|
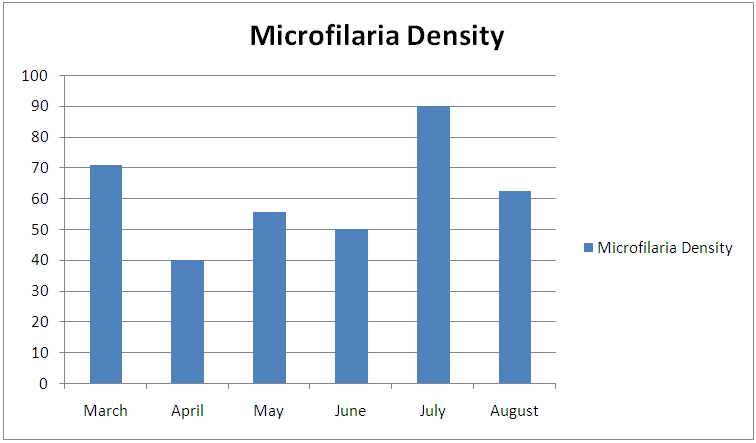 | Figure 3. Microfilariae density encountered during the study period |
Table 4. Seasonal infection rate of bovine Onchocerciasis during the study period
 |
| |
|
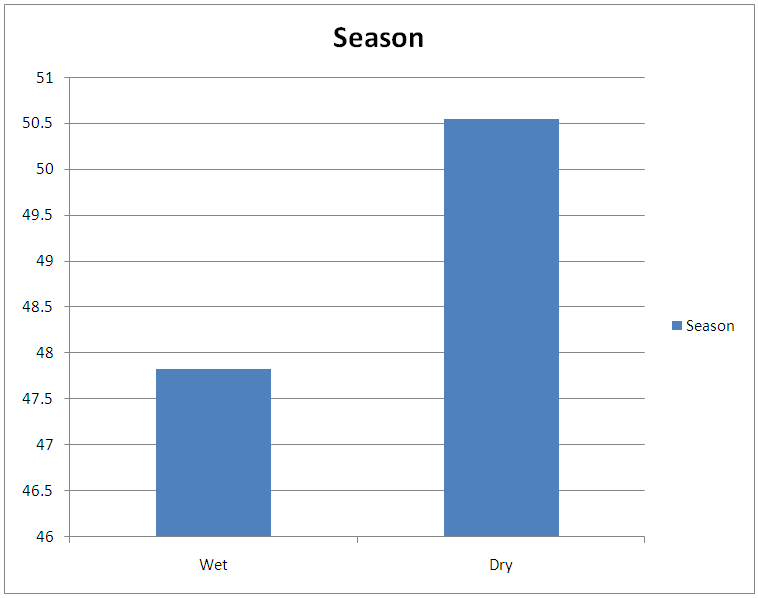 | Figure 4. Seasonal infection rate of bovine Onchocerciasis during the study period in percentage |
Table 5. Sex-related prevalence of infection during the period of study
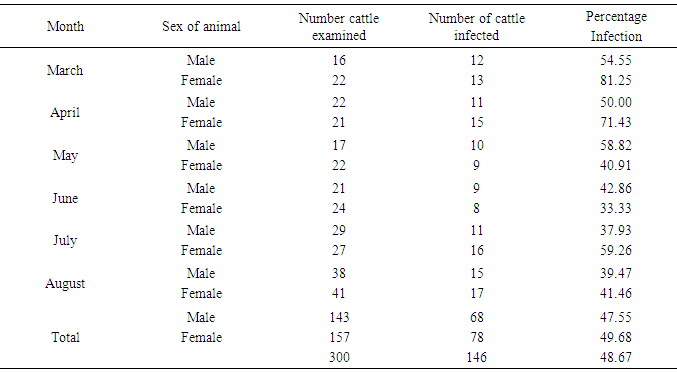 |
| |
|
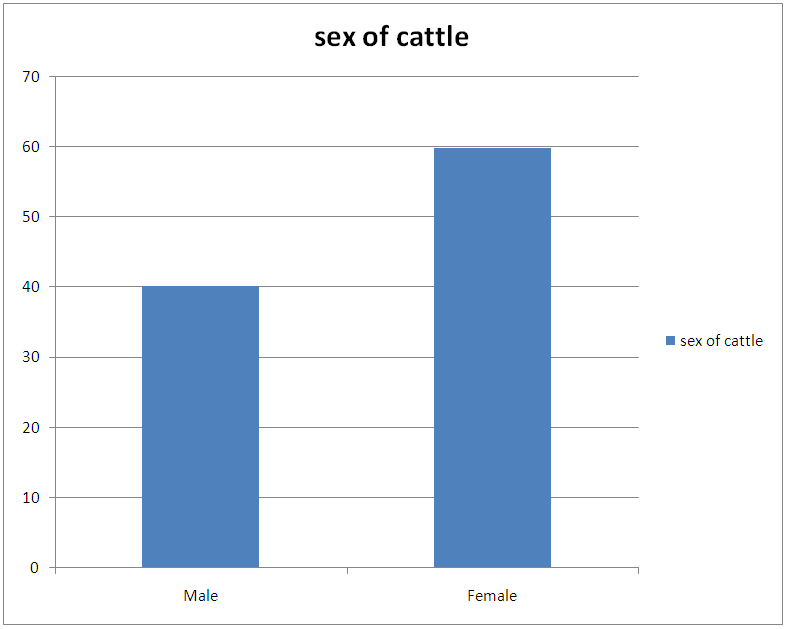 | Figure 5. Sex-related prevalence of infection during the period of study |
Table 6. An anatomical sites and infection rates of cattle examined
 |
| |
|
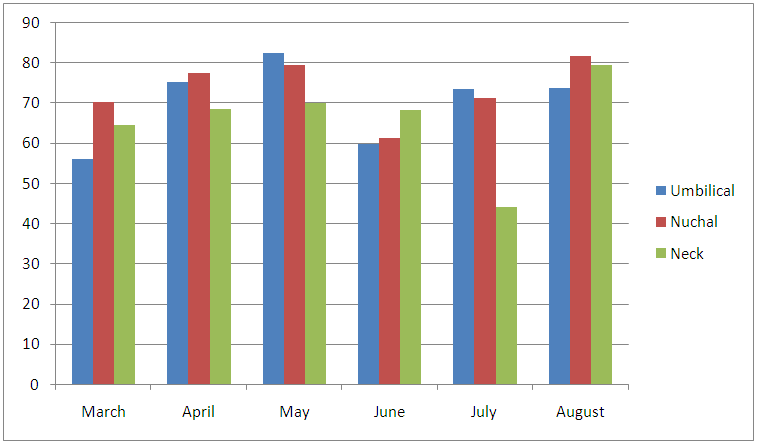 | Figure 6. An anatomical sites and infection rates of cattle examined |
PICTURES OF VARIOUS SPECIES OF Onchocerca MICROFLARIAE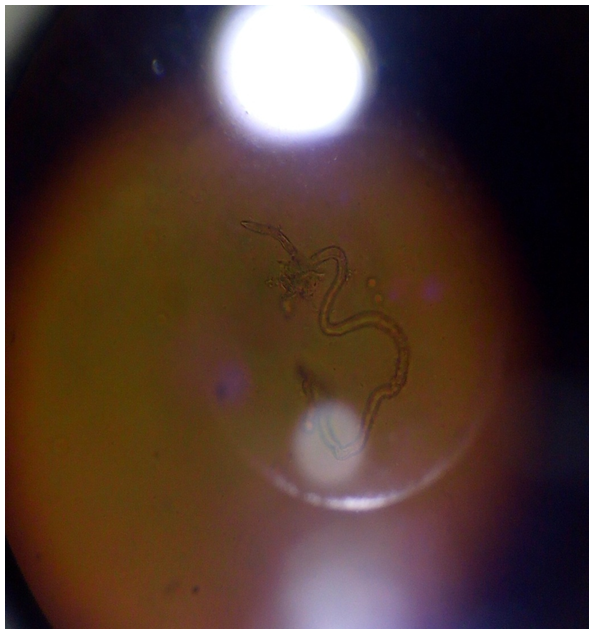 | Figure 7. Picture of Onchocerca gutturosa stained with Giemsa |
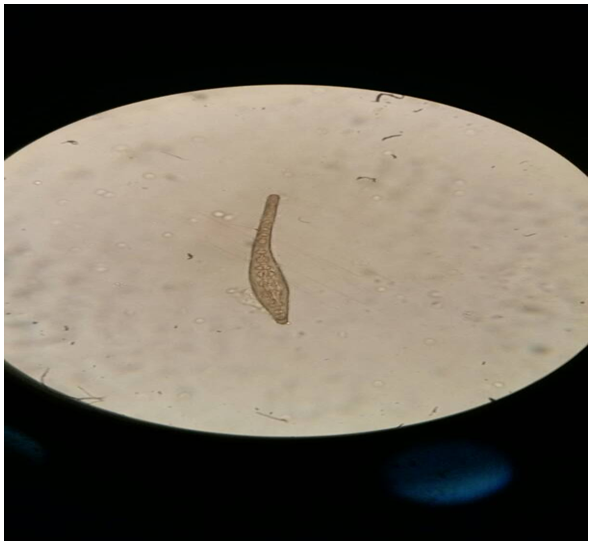 | Figure 8. Picture of Onchocerca gibsoni stained with Methylene blue |
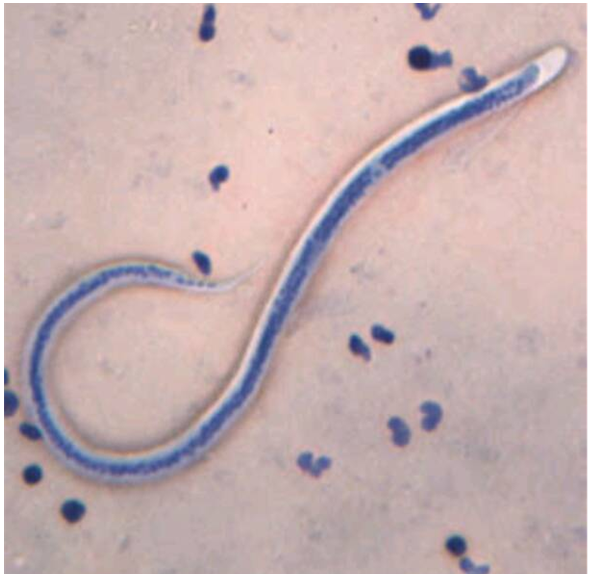 | Figure 9. Picture of Onchocerca dukei stained with 2% HCl in Methylene blue |
5. Discussion
This result also shows that more microfilariae (354) were recorded from the umbilicus. This is a confirmation of the observations by Stewards (1998). Looking at the overall microfilariae density it will be noted that an average (mean microfilariae density) of 2.03 microfilaria per 2mm2 of skin, was recorded. This is low because of the fact that some of them would have been dead before laboratory processes of incubation were affected. Of the three species of bovine Onchocerca encountered during the period of the survey Onchocerca gutturosa (63.0%) had the highest incidence, and this could be attributed to the climatic and ecological condition of the area. This is a confirmation by Crosskey (1981) that ecological and climatic condition influence the success of transmission. It was observed that parasites do not prefer any particular breed of cattle. This also agrees with the observations of Eberhard (1999) Cheema et al., (1994) in their study on Onchocerciasis among cattle in Georgia noting that breed of cattle had no effect on the prevalence of infection. This therefore was further attributed to the fact that all cattle have the same genetic makeup, hence, the vectors feed on all of them without discrimination.Considering age and the prevalence of infection it was observed that young adult animals (5-6 years) were highly parasitized than the younger ones (3-4years) as well as those (7 years and above) which had least parasitic burden. This could be attributed to the behavior of the young adult animals (5-6 years) that mate actively and are more mobile, thus, are exposed more to vector bite. Proportion of female animals found to be higher (49.68%) than male population of (47.55%). This could be attributed to the wandering habits of the male in search of female partners hence, are more exposed to the bite of Simulium species. However, it is surprising to know that male is more infected than females. Since both sexes eat and wonder in the same environment one will expect perhaps equal infection rate. Unexpectedly, this was not so. It is suspected that sex related physiological mechanism could be a contributing factor. This aspect indeed merits further study. A mixed infection rate of (23%) was recorded from different anatomical sites. This also agreed with observations by Sandground (2003) that the number of valid species in the cattle is a debatable subject, since the specific characteristic are variable. Physically it was observed that very few of the infected cattle (5 years and above) in particular, had nodules probably due to long standing Onchocerciasis. Thus, confirming Browns (2001) observation that Onchocerca lesions and nodules are usually associated with long standing Onchocerciasis. Among the three techniques of staining employed for the study, the result of (1:5000 methylene blue in physiological saline) technique was quite outstanding. Therefore, this is an advantage over the other two techniques namely: Giemsa and Fields. This is in agreement with the observation of Shape, that microfilariae Onchocerca species, Acanthocheilonema perstan and loa loa, absorbs the above stain in that concentration while Wuchereria bancrofti microfilariae are particularly inert to the action of the stain. With respect to these results, it is evidence that bovine onchocerciasis is relatively high in the study area (Karu FCT Abuja). This is attributed to continuous alteration of ecological sites for Simulium by yearly activities of farmers in bush burning, various activities which pollutes the stream.
6. Conclusions
From the study carried out, evidence showed that Bovine onchocerciasis is relatively high in the study area Karu, F.C.T from the 300 sample collected 48.66% of the various species of the microfilaria are well distributed around the anatomical sites. Climatic and ecological condition influence the success of their transmission and their densities. The breed of the cattle however had no much influence on the prevalence of infection.
References
| [1] | Cheesbrough, M District Laboratory Practice In Tropical Countries. (2001) Part 1.ISBN 0 521665469. |
| [2] | Naik, B; Onchocerciasis due to O. armilatta in cattle at Orissa. (1992) J. of Helminthol36. 313-326. |
| [3] | Kershaw, W.E Chambers, T.A; Duke, B.O.L; Studies on the intake of microfilariae by their vectors.; 2006 Ann. Trpeen parasite 48, 329-339. |
| [4] | Umeh, R.E. The causes and profile of visual loss in an onchocerciasis-endemic forest- savanna zone in Nigeria. Ophthalmic Epidemiol, 1999 Dec6(4)303-15. |
| [5] | Eberhard, M Studies on the Onchocerca found in cattle in the U.S.I systematics of O. gutturosa and O. lienalis with a description of O.stiles sp.1979. J. of Parasitol 65(3). pp379-388. |
| [6] | Copeman D. B, Ference S. A; Harty T. M; Courtney, C.H; Distribution of Onchocerca sp. Of cattle. 1997, J. vet parasite 31 pages 345-349. |
| [7] | Nelson, G.S; Eichler E. (1997) The maintenance of Onchocerca gutturosa microfilariae in invitro and in vivo” (Demonstration) Trans Roy. Soc. Trop med and Hyg 60,17. |
| [8] | Harty, H.A; Ference S. A; Harty T. M; Courtney, C.H; Copeman D. B; Distribution of Onchocerca sp. Of cattle. (1989) J. vet parasite 31 pages 345-349. |
| [9] | Davies, J.B. A review of past and present aspects of Simulium species in Mexico together with recommendations.2010. American Journal of tropical medicine. 83(1): 15-20. |
| [10] | Shastri, U.V, Shvpuje, P, R. et al; Morphological study of Onchocerca species infecting nuchal ligaments of bovine and buffaloes from Marathwada region. Indian Journal Of Parasitology (1986)10; pp271-276. |
| [11] | Kershaw, W.E; Chambers, T.A; Duke, B.O.L; Studies on the intake of microfilariae by their vectors. 2006 Ann. Trpeen parasite 48, 329-339. |
| [12] | Nelson, G.S (1990) The maintenance of Onchocerca gutturosa microfilariae in invitro and invivo” (Demonstration) Trans Roy. Soc. Trop med and Hyg 60, 17. |
| [13] | Adeleke, M.A et al Biting on human body parts of Simulium vectors and its implications for the manifestation of Onchocerca nodules along Osun River, South Western, Nigeria. J. Vector Bovine Dis 2012: 49(3): 140-2. |
| [14] | Brinkman, U.W (1994) Economic development and tropical diseases. Ann.N.Y.Acad. Sci.740. |
| [15] | Coulard, J.P et al (1983) Treatment of human Onchocerciasis with ivermectin. Bull. Soc.Patho.C Sot. Filiales. 76,681-688. [Pub.Med]. |
| [16] | Awadzi, D.B et al (2005) Evaluation of drugs against Onchocerca microfilariae in an inbreed mouse model. Tropenmed parasite. 37, 39-45. |
| [17] | Green, B.M et al (1990) Impact of mass treatment of Onchocerciasis with ivermectin on the transmission of infection. Sc 250.pp116-118. |
| [18] | Aziz, M.A et al (1990) Efficacy and tolerance of ivermectin in human onchocerciasis. Lancet (2)(171-173). [Pub.Med]. |
| [19] | Townson, S; Tagboto, S.K.; Onchocerca volvulus and Onchocerca lienalis: The microfilaricidal activity of moxidectin compared with that of ivermectin in vitro and in vivo. 1996: Ann. Trop. Med. Parasitol (5): 497-505. |
| [20] | Onwuliri, C.O.E; Eno, E. Onchocerciasis in Plateau state of Nigeria 1992; J. of Hygiene Epidemiology and Immunology 36 (2)153-160. |
| [21] | Obed, G et al (2017) Study on population dynamics of black flies imagos. 530-539. |
| [22] | Roberts, D.M; Irving-Bell, R.T. (1985) Circadian flight activity of Simulium spp (Diptera:Simuliidae) sampled with a vehicle-mounted net in Central Nigeria. .1985: Ann. Trop. Med. Parasitol (6): (507-552). |
| [23] | Anosike, J.C et. al Studies on filariasis in Bauchi State Nigeria. Endemicity of Human Onchocerciasis in Ningi Local Government Area. (1992) Ann Trop Med Parasitol Feb; 89(1): 31-8. |
| [24] | Steward, J.S The occurrence of Onchocerca gutturosa (Neumann) in cattle in England with an account of its life history and development in S. ornatum. 1998 Parasitology 29, 212-219. |
| [25] | Gnedina, M.P (1950) Biology of the nematode O.gutturosa parasitic in cattle. Dohl. Akad. Nauk. S.S.S.R, 70, 169-171. |
| [26] | Crosskey, R.W. A review of Simulium damnosum and human onchocerciasis in Nigeria, with special reference to geographical distribution and the development of a Nigerian National Control Campaign (1981). Tropenmed Parasitol 32(1): 2-16. |
| [27] | Cheema, A.H et al (1998); “Bovine Onchocerciasis caused by Onchocerca armilatta” 1994 Inst.ann. husb and vet .sci. Human, China 17 (2) 103-108. |
| [28] | Sandground, J.H. (1994) Nodule diseases due to various species of Bovine Onchocerca. A.M.J.Ani and Hyg 11.31-60. |
| [29] | Brown, R. M; Studies on nodule disease of cattle. 1989 J. Parasite 52 (1) 286-287. |





 λ2 Tabulated = 25.00Calculated λ2 is greater than tabulated λ2 so there was significant difference in the prevalence of infection at the course of this research work. (P<0.05)
λ2 Tabulated = 25.00Calculated λ2 is greater than tabulated λ2 so there was significant difference in the prevalence of infection at the course of this research work. (P<0.05)






 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML





