-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2019; 9(3): 125-129
doi:10.5923/j.ajmms.20190903.10

Learning Efficiency and Possibility of Anti-Leukotriene Preparations for Children with a Bronchial Asthma in Uzbekistan Conditions
Mirrahimova Maktuba Habibullayevna1, Khalmatova Barno Turdihadjayevna2, Tashmatova Gulnoza Aloyevna3
1Associate Professor of the Department of Children's Diseases №1, Tashkent Medical Academy, Uzbekistan
2Professor, Head of the Department of Children's Diseases №1, Tashkent Medical Academy, Uzbekistan
3Assistant of the Department of Children's Diseases №1, Tashkent Medical Academy, Uzbekistan
Correspondence to: Tashmatova Gulnoza Aloyevna, Assistant of the Department of Children's Diseases №1, Tashkent Medical Academy, Uzbekistan.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

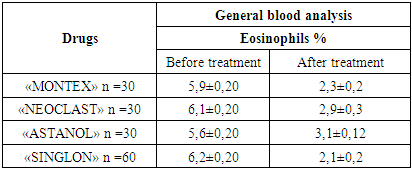
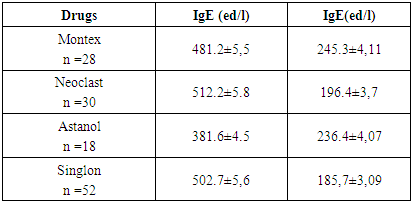
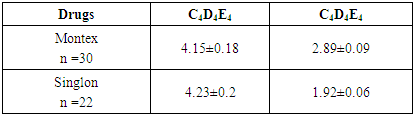
The article presents data from a clinical trial to study the efficacy and tolerability of local production montelukast drugs. The clinical efficacy of the drugs was evaluated in points to improve clinical and laboratory data. The study showed positive dynamics of clinical and laboratory parameters in all children who received Montelukast drugs. Studies have shown that taking montelukast drugs leads to a decrease in the level of cys-LT, IgE in children with bronchial asthma.
Keywords: Children, Allergies, Atopy, Bronchial asthma, Montelukast, Randomized clinical trial
Cite this paper: Mirrahimova Maktuba Habibullayevna, Khalmatova Barno Turdihadjayevna, Tashmatova Gulnoza Aloyevna, Learning Efficiency and Possibility of Anti-Leukotriene Preparations for Children with a Bronchial Asthma in Uzbekistan Conditions, American Journal of Medicine and Medical Sciences, Vol. 9 No. 3, 2019, pp. 125-129. doi: 10.5923/j.ajmms.20190903.10.
Article Outline
1. Introduction
- All over the world, including in Uzbekistan, childhood asthma is a chronic allergic disease that requires special attention. Epidemiological studies show that almost 1/3 of sick children suffer 5 or more wheezing episodes per year (which is considered as the equivalent of an attack in adults). According to the WHO, the prevalence of BA has reached 5% of the average adult and 10% among the world's children [1, 4, 8, 10, 12]. Bronchial asthma often develops among children of preschool age (80%), often the first attacks occur in the first year of life. Despite certain successes achieved in developing new approaches to the prevention and treatment of this pathology, the prognosis of asthma is not comforting, its further growth is expected, especially among children, an increase in the number of severe forms and fatal cases, which is associated with a continuing negative effect on the human body pollutants, ozone and aeroallergens with genetic prerequisites [2, 5, 9, 15].Important mediators involved in the pathogenesis of the ABA are cysteinyl leukotrienes (Cys-LT) (LTC4, LTD4, LTE4), which have pronounced pro-inflammatory and bronchoconstrictor properties and are formed as a result of arachidonic acid exchange via the 5-lipoxygenase (5-LO) pathway from phospholipid hypophospholipid hypophospholipid phosphate gland in the phospholipid hypophospholipid gland by the action of phospholipase A2. The increase in their concentration in the blood causes bronchospasm, increased secretion of bronchial glands, increased vascular permeability [7, 9, 11, 14]. Therefore, the use of pharmacological drugs - antagonists of cis-LT1 receptors has been approved for a long time in many countries of the world and well-proven pharmacotherapy of BA and AR in adults and children [3, 4, 6, 9, 13].In the pharmaceutical market of the Republic of Uzbekistan today there are several names of Montelukast drugs (Singlon, Gedeon Rixter, Hungary; Montular, Kusum, India; Mitekа, India; Brizezi, India, etc.).
2. Purpose of the Study
- Evaluation of the clinical efficacy and tolerability of montelukast preparations Uzbekistan, in comparison with the drug Singlon, produced by Gedeon Richter (Hungary).
3. Materials and Methods
- The study was conducted in accordance with the Hel ¬ Sync Declaration (adopted in June 1964 (Hel - sinki, Finland) and revised in October 2000 (Edinburgh, Scotland)) and in accordance with the Law of the Republic of Uzbekistan "On Drugs and Pharmaceutical Activities "," The National Standard of Uzbekistan - GSR - Good Clinical Practice ", taking into account the GSR rules applied in international practice, the Regulation" On the procedure for conducting clinical trials and the examination of clinical research materials medicines and medicines” (Appendix 1 to the Order of the Ministry of Health of the Republic of Uzbekistan No. 40 of January 26, 2015). From parents of patients obtained informed consent. The clinical trial was limited, comparative, open, controlled, randomized with four parallel groups. Studies have been conducted in patients who are hospitalized in the departments of pulmonology, allergology at the Department of Children’s Diseases No.1 of the Tashkent Medical Academy.The study involved 150 children aged 2 to 14 years with a diagnosis of intermittent, mild and moderate persistent bronchial asthma. All children were divided into 4 groups: 1st group - children who received Astanol (Remedy Group, Uzbekistan) n=30; Group 2 - children who received the Neoclast (OOO Nobel) n=30; Group 3, children who received Montex (Nika Pharm, Uzbekistan) n=20; Group 4 (control) children who received the drug Singlon (Gedeon Rixter, Hungary) n=60. The average age of children was 6.8 ± 2.1 years. The number of boys and girls included in the study was comparable. Children from 2 to 5 years were prescribed montelukast in a dose of 4 mg (chewable tablets), children from 6 to 14 years old 5 mg (chewable tablets) once a day, for the night, for 1 month. All children underwent a general clinical examination (collection of allergological anamnesis, examination, physical) examination, complete blood count, determination of total IgE, determination of cys-LT in the urine, chest X-ray, spirometry or peak flowmetry and after the study.Urine samples for the determination of cys-LT in an amount of 5 ml were collected in the morning. The quantitative determination of the final metabolite cys-LT (LTC4/D4/E4) in the urine (reagent from Neogen, Ukraine) was performed by enzyme immunoassay. Measurement range: 0.04-8 ng / ml. Sensitivity: 0.04 ng/ml. Statistical processing of the results was performed using the Statistica 10.0 software package. Data are presented as arithmetic means with an average error. The difference of values was considered significant at p <0.05.
4. Results and Discussion
- Criteria for inclusion in the test were:- patients of both sexes aged from 2 to 14 years;- diagnosis – intermittent, mild and moderately severe persistent bronchial asthma;- availability of informed written consent of the patient’s parents (guardians) for the child’s participation in the clinical trial.The criteria for non-inclusion were:- age of patients younger than 2 years and older than 14 years;- the presence of contraindications for the appointment of Montelukast;- severe bronchial asthma constant flow (because monotherapy is not recommended). - Patient participation in other clinical studies in the past 30 days;- lack of informed written consent of the patient to participate in a clinical study.The effectiveness of the drugs was evaluated by the following criteria:- clinical improvement of the patient's condition (taking into account the dynamics of characteristic manifestations);- reduction of manifestations and intensity of shortness of breath, cough, sputum.- Improving laboratory data.Evaluation of the effectiveness of the studied drug was carried out on the basis of the above criteria in points according to the following scale:
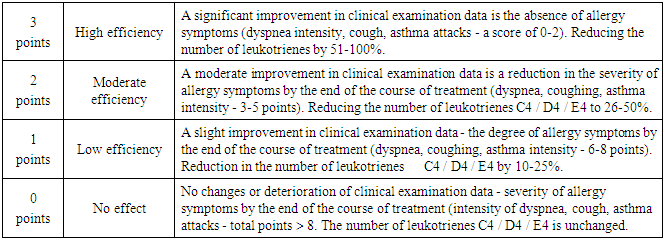 Tolerability of the drug was evaluated on the basis of subjective symptoms and sensations, which the patient or his parents reported independently and taking into account the objective data obtained by the doctor. Taking into account the dynamics of laboratory parameters, as well as the frequency of occurrence and the nature of adverse reactions. Assessment of the tolerability of the studied drug was carried out on the basis of the above criteria in points on a scale from 0 to 4 points.
Tolerability of the drug was evaluated on the basis of subjective symptoms and sensations, which the patient or his parents reported independently and taking into account the objective data obtained by the doctor. Taking into account the dynamics of laboratory parameters, as well as the frequency of occurrence and the nature of adverse reactions. Assessment of the tolerability of the studied drug was carried out on the basis of the above criteria in points on a scale from 0 to 4 points. A simple randomization method was used to distribute the subjects into groups. The original table of patient distribution by groups was formed on the basis of random numbers obtained using the MSExcel random number generation function.The distribution by groups was carried out on the basis of sealed envelopes provided by the sponsors. After the patient was included in the study and assigned a serial number, the envelope corresponding to that number was opened, and the treatment attached to this envelope was prescribed.The starting point of the beginning of participation of the patient in the study: the day of the first dose of the study drug or the reference drug.Treatment was described in detail in all patients included in the study.Any therapy associated with concomitant diseases was registered in the history of the disease and individual registration form.All patient examination data was recorded in the patient’s history, outpatient card and individual patient registration form.
A simple randomization method was used to distribute the subjects into groups. The original table of patient distribution by groups was formed on the basis of random numbers obtained using the MSExcel random number generation function.The distribution by groups was carried out on the basis of sealed envelopes provided by the sponsors. After the patient was included in the study and assigned a serial number, the envelope corresponding to that number was opened, and the treatment attached to this envelope was prescribed.The starting point of the beginning of participation of the patient in the study: the day of the first dose of the study drug or the reference drug.Treatment was described in detail in all patients included in the study.Any therapy associated with concomitant diseases was registered in the history of the disease and individual registration form.All patient examination data was recorded in the patient’s history, outpatient card and individual patient registration form.
|
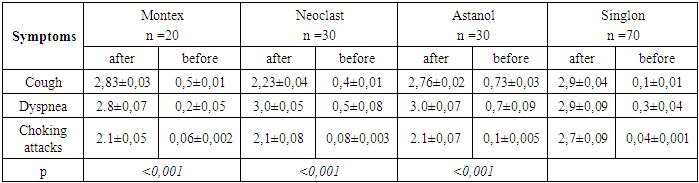
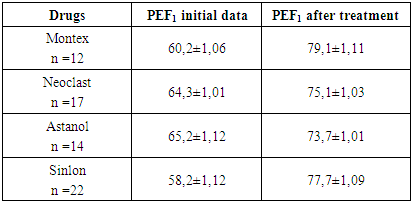
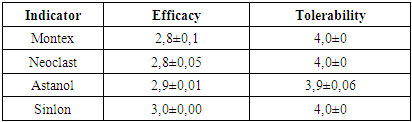
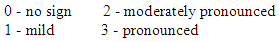 This table shows that after the use of montelukast drugs, both domestically produced and Singlelon, the clinical manifestations of allergy decreased. Only such a symptom of the disease as cough was observed in 20-30% of children, in the form of coughing.
This table shows that after the use of montelukast drugs, both domestically produced and Singlelon, the clinical manifestations of allergy decreased. Only such a symptom of the disease as cough was observed in 20-30% of children, in the form of coughing.
|
|
|
|
|
5. Conclusions
- In accordance with the results of clinical studies and recommendations of international documents (GINA, 2015) [4], montelukast recommendation for use in asthma mild to moderate severity as an alternative to inhaled corticosteroids or as an adjunctive therapy. The use of montelukast in pediatric practice will ensure the stability of the state of children with bronchial asthma, and allows to reduce the dose of IGS.The data obtained allow us to conclude that the drugs of monteukast production Uzbekistan (Montex, Astanol, Neoclast) are effective drugs for the treatment of children with intermittent and persistent form of asthma (mild and moderate). With a light course of bronchial therapy in children from 2 to 5 years of age, monotelukast monotherapy can be used.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML