-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2019; 9(3): 114-124
doi:10.5923/j.ajmms.20190903.09

Update on Diagnosis and Management of Early Stage Diabetic Kidney Disease
Sheikh Salahuddin Ahmed
Department of Internal Medicine, Faculty of Medicine and Defense Health, National Defense University of Malaysia, Kuala Lumpur, Malaysia
Correspondence to: Sheikh Salahuddin Ahmed, Department of Internal Medicine, Faculty of Medicine and Defense Health, National Defense University of Malaysia, Kuala Lumpur, Malaysia.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Diabetes mellitus is the most common cause of end stage kidney disease (ESKD) that needs renal replacement therapy (dialysis or kidney transplantation) to sustain life. There is also increased cardiovascular morbidity and mortality in patients with diabetic kidney disease (DKD). In this review article, the diagnosis and the updated different approaches used in the management of early stage DKD has been focused. Various international/ national guidelines and relevant literatures that were published through January 2001 to January 2019 for the recommendations on the diagnosis and management of DKD were reviewed. The benefits of multifactorial interventions in the management of people with early stage DKD to prevent and slow the progression to ESKD, has been clearly demonstrated. Recent studies reveal that some antidiabetic drugs have renoprotective effect evidenced by reduction in albuminuria and improvement in renal function; therefore, they are currently recommended in the management of DKD.
Keywords: Diabetic kidney disease, Diabetic nephropathy, Diagnosis, Management, Multifactorial approach, Update
Cite this paper: Sheikh Salahuddin Ahmed, Update on Diagnosis and Management of Early Stage Diabetic Kidney Disease, American Journal of Medicine and Medical Sciences, Vol. 9 No. 3, 2019, pp. 114-124. doi: 10.5923/j.ajmms.20190903.09.
Article Outline
1. Introduction
- Diabetes mellitus (DM) is the leading cause of chronic kidney disease (CKD) worldwide. CKD occurs in 20–40% of patients with patients suffering from diabetes [1]. A number of patients with diabetic kidney disease (DKD) ultimately culminate into end stage kidney disease (ESKD). To sustain life, the treatment of ESKD needs renal replacement therapy (RRT) which includes dialysis or kidney transplantation. Dialysis & kidney transplantations are not free of complications; the quality of life of those persons on dialysis or kidney transplantation is reduced. The prevalence of ESKD due to diabetes is increasing due to continued rise in the number of type 2 diabetes (T2D) worldwide. DKD is also associated with an increased risk for cardiovascular (CV) morbidity and mortality (i.e., angina, unstable angina, myocardial infarction, peripheral arterial disease, stroke and death) [2]. There is strong evidence that a number of interventions if initiated at an early stage of DKD, reduce the risk and slow the progression to ESKD, and decrease CV morbidity and mortality. Therefore, early diagnosis and management of DKD is important.
2. Objectives
- The current method of diagnosis and updated interventions in management of early stage DKD will be the focus of this presentation. The management of those factors responsible for development and progression to ESKD and CV events have been discussed. The management of complications of DKD, and that of advanced DKD or ESKD is out of scope in this review article. Some controversies in the diagnosis and management of DKD have also been delivered.
3. Materials and Methods
- Various international/ national guidelines and relevant literatures that were published through January 2001 to January 2019 for the recommendations on the diagnosis and management of DKD were reviewed.
4. Definition of Diabetic Kidney Disease
- The term DKD is used to describe CKD resulting from diabetes [3]. In 2002, National Kidney Foundation (NKF), a United States (US) health organization, defined CKD as either kidney damage or glomerular filtration rate (GFR) <60 mL/min/1.73m2 persisting for 3 months or more irrespective of the etiology [4]. CKD has been grouped into 5 stages by estimated glomerular filtration rate (eGFR). NKF, defined categories of decreased eGFR as mild (stage 2, eGFR 60 to 89 mL/min/1.73m2), moderate (stage 3, eGFR 30 to 59 mL/min/1.73m2), severe (stage 4, eGFR 15 to 29 mL/min/1.73m2) and ESKD/ kidney failure (stage 5, eGFR <15 mL/min/1.73m2). Microalbuminuria has been defined as: (i) excretion of 30-300 mg of albumin in a 24-hour urine collection sample, or (ii) urinary albumin excretion rate (AER) of 20-200 μg/min in a timed collection of urine, or (iii) urinary albumin creatinine ratio (ACR) of 30-300 mg/gm without regard to age & sex in a random or spot sample of urine [3]. Albuminuria values >300 mg/24 hour has been defined as macroalbuminuria. In 2012 the Kidney Disease: Improving Global Outcomes (KDIGO), a foundation governed by an international board, revised the definition of CKD and made certain changes [5]. CKD is now defined as presence of kidney damage or impairment of kidney function persisting for >3 months with implications for health. The addition of ‘with implications for health’ has been intended to reflect that some abnormalities of kidney structure or function may not have any clinical significance (e.g., simple renal cysts). The classification of CKD is based on three variables (CGA): (i) cause of CKD, (ii) GFR category (G1-G5), and (iii) albuminuria category (A1, A2 & A3). Previously CKD was grouped into 5 stages based on eGFR [4]. However, the risk of worsening of kidney function is found closely linked to the amount of albuminuria, and so it has been incorporated into the new classification. The term microalbuminuria/ macroalbuminuria has been eliminated and replaced by albuminuria categorizing by specific values. Albuminuria has been grouped into 3 categories: (i) category A1 represents normal or mild (<30 mg/24 hour) albuminuria, (ii) A2 with moderate (30-300 mg/24 hour) albuminuria and (iii) A3 having severe (>300 mg/24 hour) albuminuria. The threshold for albuminuria of >30 mg/24 hour has been chosen as one of the important marker of kidney damage to indicate CKD based on a meta-analysis that demonstrated associations of albuminuria >30 mg/24 hour with subsequent risk of all-cause and CV mortality, CKD progression and kidney failure in general population as well as patients with kidney disease. The threshold values of G1-G5 are same as the CKD stages 1–5 recommended previously by NKF in 2002 [4]. GFR category 3, has been further refined into 3a and 3b, based on substantial data that there are differences in outcomes and risk for those who have GFR values 45—59 versus 30—44 mL/min/1.73m2. In 2014, NKF in their commentary has approved the new definition of CKD as well as the threshold of albuminuria (of >30 mg/24 hour) as a marker for kidney damage [6]. The new definition and classification of CKD is now becoming popular and recently has been accepted by various organizations and incorporated in their guidelines.In this paper, we have considered (i) early stage of DKD in those patients with diabetes having normal or mildly decreased eGFR (>60 mL/min/1.73m2) with microalbuminuria or moderate albuminuria (30-300 mg/24 hour) having no other cause, and (ii) advanced DKD having eGFR <30 mL/min/1.73m2 [7].
5. Diagnosis of Diabetic Kidney Disease
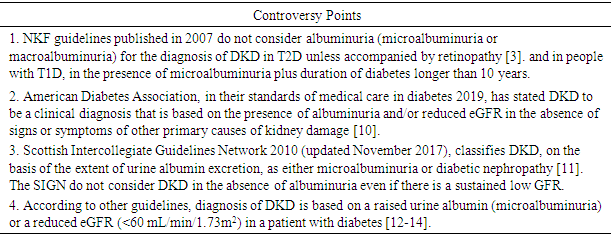
- The diagnosis of DKD is based on the presence of albuminuria and/or reduced eGFR (<60 mL/min/1.73m2) persisting for 3 months or more, in the absence of signs or symptoms of other causes of kidney disease [8]. DKD, traditionally termed “diabetic nephropathy,” is a clinical diagnosis that historically has been based on the findings of albuminuria with no other cause, in a person with diabetes. The term diabetic glomerulopathy is reserved for biopsy-proven kidney disease caused by diabetes [3]. As biopsy will not alter the management of DKD which has been diagnosed clinically, it is routinely not done. However, people with diabetes may develop kidney disease for other reasons not related to diabetes. A kidney biopsy may be required in some patients with diabetes and CKD to determine the underlying cause of the kidney disease in situations like rapidly declining kidney function, increasing proteinuria (particularly if albuminuria is in nephrotic range), active urinary sediment, resistant hypertension, or evidence of other systemic disease raising concerns about nondiabetic glomerular disease [3]. The criteria for early diagnosis of DKD vary among different guidelines [8]. 5.1 NKF guidelines published in 2007 do not consider albuminuria (microalbuminuria or macroalbuminuria) for the diagnosis of DKD in T2D unless accompanied by retinopathy [3]. In most people with diabetes, CKD should be considered in the presence of (i) macroalbuminuria or microalbuminuria plus retinopathy, and (ii) in people with type 1 diabetes (T1D), in the presence of microalbuminuria plus duration of diabetes longer than 10 years. Based on studies showing positive relationship between the duration of diabetes and DKD, they expressed that the presence of elevated albuminuria in T1D of short duration should raise possibilities about non-DKD [3]. In 2012, NKF has expressed concern that the presence of macroalbuminuria without retinopathy, present within 10 years of onset in patients with T1D, suggests a need for investigations to rule out non-DKD; as there is spontaneous remission of microalbuminuria in up to 40% of patients with T1D [9]. About 30–40% remains with microalbuminuria and do not progress to macroalbuminuria over 5–10 years of follow-up.5.2 American Diabetes Association (ADA), in their standards of medical care in diabetes 2019, has stated DKD to be a clinical diagnosis that is based on the presence of albuminuria and/or reduced eGFR in the absence of signs or symptoms of other primary causes of kidney damage [10]. However, signs of CKD may be present at diagnosis or without retinopathy in T2D, and reduced eGFR without albuminuria has been frequently reported in type 1 and T2D and is becoming more common over time as the prevalence of diabetes increases in the US [10].5.3 Scottish Intercollegiate Guidelines Network (SIGN) 2010 (updated November 2017), classifies DKD, on the basis of the extent of urine albumin excretion, as either microalbuminuria or diabetic nephropathy [11]. They consider microalbuminuria as the earliest, clinically detectable manifestation of classic DKD. Remission of microalbuminuria may occur and so the presence of microalbuminuria does not imply an inexorable progression to diabetic nephropathy. Diabetic nephropathy is defined by a raised urinary albumin excretion of >300 mg/day. This represents a more severe and established form of renal disease and is more predictive of total mortality, CV events and ESKD than microalbuminuria. The SIGN do not consider DKD in the absence of albuminuria even if there is a sustained low GFR. 5.4 According to National Institute for Health and Care Excellency (NICE) guidelines of United Kingdom 2014 (modified on March 2015), CKD has been defined as abnormalities of kidney function or structure present for more than 3 months, with implications for health [12]. In people with diabetes microalbuminuria should be considered clinically significant. 5.5 International Diabetes Federation (IDF) guideline, diagnose DKD on the basis of a raised urine albumin (microalbuminuria or ACR >30 mg/gm) or a reduced eGFR (<60 mL/min/1.73m2) in a patient with diabetes [13, 14].5.6 According to Malaysian guidelines 2015, the diagnosis of DKD is made clinically by the presence of persistent proteinuria (either microalbuminuria or macroalbuminuria) [15]. Microalbuminuria has been considered as the earliest sign of diabetic nephropathy as it predicts increased CV mortality and morbidity and ESKD.
6. Screening for Diabetic Kidney Disease
- Screening for DKD should be performed annually from the onset in T2D and 5 years after onset in T1D [3]. It has been reported that up to 25% of newly diagnosed patients with T2D already have microvascular complications, and there is often a 4 to 7-year time lag between the onset and the diagnosis of T2D [16]. Microalbuminuria indicating DKD rarely occurs with short duration of T1D [17]. ADA in their standards of medical care in diabetes 2019, also describes screening in all patients of diabetes with comorbid hypertension [10]. Screening is important for early diagnosis, staging and monitoring progression of DKD. Two investigations are done for screening (i) urine test for albuminuria and (ii) serum creatinine and estimation of GFR. For diagnosing DKD, albuminuria can be determined by spot urine test for ACR, or timed collection of urine to see albumin excretion rate. ACR is now, the recommended method for screening microalbuminuria in people with diabetes. Measurement of ACR in a random urine sample is often found to be easiest method to carry out by patients. ACR is best measured on an early morning sample of urine. Studies have shown that ACR measured in early morning samples correlates closely with 24-hour albuminuria [18]. An early morning sample also excludes orthostatic (postural) proteinuria. Urine screening for albuminuria should not be done during intercurrent illness or in presence of other confounding factors related to proteinuria (e.g., urinary tract infection, congestive heart failure, acute febrile illness, menstruation or vaginal discharge, exercise within 24 hours, marked hyperglycemia, marked hypertension and high protein diet). The best possible metabolic control of diabetes should be achieved before investigating patients for microalbuminuria. For diagnosing DKD, serum creatinine and eGFR should be done in all DM patients regardless of presence of albumin in urine. This is because; a significant proportion of people with T2D may have or develop CKD in the absence of albuminuria. For reporting eGFR in adults the “Chronic Kidney Disease Epidemiology” (CKD-EPI) collaboration equation has been recommended by KDIGO 2012, because it was found to be more accurate than other equations [5].
7. Risk Factors for Diabetic Kidney Disease
- The risk factors that promote the development and progression of DKD include [19]:a. Gender (male at greater risk than female)b. Age (advanced age)c. Genetic factors d. Race (e.g. Asians have higher prevalence)e. Long duration of diabetes f. Poor glycemic control g. Presence of hypertension h. Albuminuriai. Presence of other microvascular complications (e.g. retinopathy) j. Obesity k. Dyslipidemia l. Smoking
8. Multifactorial Interventions to Prevent or Delay the Progression of Early Stage DKD to ESKD
- The aim of management of early stage DKD is to delay its progression to ESKD and to reduce CV morbidity and mortality. The interventions need to be focused on those factors seen to be associated with the progression of early stage DKD to ESKD and CV events as evidenced by various studies. The benefits of a multifactorial approach in the management of people with early stage DKD has been clearly demonstrated [20]. The combination of improved glycemic control, blood pressure (BP) control, albuminuria reduction, lipid lowering, aspirin, smoking cessation, exercise and dietary intervention reduces the progression of early DKD to ESKD and also prevents CV events. Multifactorial intervention has synergistic efficacy, e.g. between blood glucose and BP control [20]. The combination of albuminuria reduction with BP lowering produces the greatest risk reduction for ESKD. Therefore, people with diabetes and microalbuminuria should be treated with a multifactorial intervention approach.
8.1. Optimum Glycemic Control
- Poor glycemic control is an independent risk factor for the development and progression of DKD. Studies have shown that increased glycosylated hemoglobin (HbA1c) level correlates with kidney damage and loss of renal function; optimum glycemic control prevents the onset and delays the progression of kidney disease in both type 1 and T2D [21-24]. Therefore, in diabetes, glycemic control should be optimised to prevent the development or delay the progression of DKD. The HbA1c target for non-pregnant adults that should prevent the progression of the early stage DKD to ESKD has been recommended by various guidelines varies from ≤6.0 to 7.0% [25-28]. There is evidence that HbA1c target of ≤6.5—7.0% reduces the development or progression of albuminuria, the rate of decrease in GFR and microvascular complications of diabetes [21-23, 29]. HbA1c level should be interpreted carefully keeping in mind the confounding factors like kidney disease, anaemia and altered red cell survival [30]. However, there are insufficient data and trials regarding the ideal glycemic target in patients with eGFR <60 mL/min/1.73m2 (CKD stage 3 or more). Intensive or tight glycemic control is associated with higher risk of hypoglycemia and hypoglycaemia related CV events in patients with advanced DKD, especially who are elderly, with a history of severe hypoglycaemia, poor glycemic control at baseline, having substantial comorbidities and receiving insulin or sulphonylureas [21-23]. In those patients, intensive glycemic control for achieving a HbA1c target of ≤6.5—7.0%, has not been shown to diminish the progression of DKD or CV events; so guidelines have recommended less stringent HbA1c goals (such as <8%) [25, 26, 28]. For non-pregnant adults having T2D with short duration, managed either by lifestyle and diet, or combined with a single drug without significant hypoglycaemia, having a long life expectancy, or no significant cardiovascular disease (CVD), guidelines recommend HbA1c target of 6.5% or less [25-27]. Therefore, HbA1c target should be personalized that is based on age, life expectancy, duration of diabetes, risk of hypoglycaemia and presence of associated comorbidities.It should be kept in mind that all the antidiabetic drugs are not absolutely safe in a patient having DKD; they are at increased risk of hypoglycemia and also other adverse effects of antidiabetic drugs at standard doses [31]. This is due to impaired renal gluconeogenesis from lowered kidney mass and impaired metabolism/ clearance of some antidiabetic drugs. For safe use, renal factors should be considered when selecting antidiabetic medications for individual patients with DKD. For patients with eGFR <60 mL/min/1.73m2, (CKD stage 3) certain drug dose adjustment or discontinuation may be required to avoid the risk of hypoglycemia and adverse effects [1]. Among the other factors considered in choosing antidiabetic drugs include CV comorbidity, impact on weight, cost, risk for side effects and patient preferences. Recently, some new drug options have become good alternatives in the management of DKD as they have beneficial effect in preventing development and progression of DKD. Several current trials have indicated the renoprotective effect of sodium-glucose cotransporter 2 (SGLT2) inhibitors (dapagliflozin, canagliflozin, and empagliflozin), and glucagon like peptide-1 (GLP-1) receptor agonists (liraglutide and semaglutide) [32-35]. They have been reported to improve renal outcomes by reducing the amount of albuminuria and preventing GFR loss in patients with T2D. Therefore, in patients with DKD, SGLT2 inhibitor or/and a GLP-1 receptor agonist use is considered that has shown to reduce risk of CKD progression, CV events, or both.
8.2. Optimum Blood Pressure Control
- Uncontrolled BP is a strong risk factor for the development of albuminuria and deterioration of renal function with progression of DKD to ESKD. Hypertension aggravates CV morbidity and mortality in DKD. There is firm evidence that the optimum control of BP can reduce the development and progression of DKD [36]. BP lowering also reduces CV events in people with DM. Angiotensin-converting enzyme inhibitors (ACEIs)/ angiotensin receptor blockers (ARBs) are the preferred fist-line therapy in DKD because they have additional renoprotective effect apart from BP reduction. Studies have shown that in hypertensive patients with DM, ACEIs/ARBs can reduce the level of albuminuria and the rate of progression of renal disease to a greater degree than other antihypertensive agents that lower BP by an equal amount [36-38]. The ACEIs/ARBs, have also shown to reduce major CV events in patients with diabetes, thus further supporting the use of these agents in patients with albuminuria, a CV risk factor [38]. In the treatment of the non-pregnant diabetic patient with hypertension, either ACEIs or ARBs should be used. If one class is not tolerated, the other should be substituted (e.g. if cough is associated with ACEIs then it should be switched to ARBs). Other drugs, such as diuretics, calcium channel blockers and β-blockers, should be used as additional therapy to further lower BP in patients already treated with ACEIs/ARBs, or as alternate therapy in the rare individual unable to tolerate ACEIs/ARBs. In the initiation of ACEI /ARB therapy, some patients with CKD may have an initial decrease in GFR, a mild increase in the serum creatinine and a mild increase in the potassium level [39]. Therefore, serum creatinine and potassium levels should be measured and the GFR should be estimated before starting ACEI/ARB therapy in DKD. These measurements should be repeated between 1 and 2 weeks after starting ACEI/ARB therapy and after each dose increments [12]. Mineralocorticoid receptor antagonists (spironolactone, eplerenone, and finerenone) in combination with ACEIs or ARBs are effective for management of resistant hypertension but with an increase in hyperkalaemic episodes, hypotension and acute kidney injury (AKI) and therefore not recommended [25]. In 2012, KDIGO had recommended BP target of <130/80 mmHg if hypertension in diabetes is associated with albuminuria ≥30 mg/day, and <140/90 if albuminuria <30 mg/day [5]. In 2014, for adult patients with DM having CKD, panel members of Joint National Committee (JNC) 8, has recommended BP target of <140/90 mmHg without differentiation by levels of proteinuria [40]. Recent recommendations advocate individualised BP targets; less stringent goals may be considered for frail patients with complicated comorbidities or those who have adverse medication effects [41]. Lower targets, such as <130/80 mmHg or <120/80 mmHg, may be appropriate for certain individuals, such as younger patients, or in patients with a high CV risk, if they can be achieved safely without undue treatment burden or side effects of therapy [41, 42]. For individuals with diabetes and hypertension at lower risk for CVD, a BP target of <140/90 mmHg has been recommended by ADA in their standards of care in diabetes, 2019 [42]. ACEIs/ARBs are the preferred drugs in T2D and are used in the treatment of hypertension, CKD and cardiac failure. These drugs have been shown to be relatively safe in the short term. Recently, concerns have raised that the long term use of ACEIs may be associated with increased risk of lung cancer [43]. A recent cohort study revealed that the use of ACEI was associated with an increased risk of lung cancer compared with use of ARBs [43]. The association was particularly elevated among people using ACEIs for more than five years. The authors concluded by recommending additional studies with long term follow-up to investigate the effects of these drugs on incidence of lung cancer.
8.3. Limitation of Excess Protein Intake
- The role of dietary protein restriction in slowing progression of CKD is the subject of debate. Some studies in patients with varying stages of DKD have shown that the limitation of excess dietary protein slows the progression of albuminuria, GFR decline and occurrence of ESKD [44-46]. Other trials conducted in people with DKD did not demonstrate a beneficial effect of protein restriction in delaying disease progression [47-49]. Advanced CKD is associated with a protein wasting syndrome which is directly correlated with morbidity and mortality. Protein restriction is not advisable in those with evidence of or at risk of malnutrition [5]. In stage 4 & 5 CKD (nondialysis-dependant), KDIGO 2012 and ADA 2019 guidelines, recommend daily protein allowance of 0.8 gm/kg [5, 10]. In stage 1-3 CKD, KDIGO 2012 guideline recommends protein intake up to 1.3 gm/kg/day [5]. Dietary protein restrictions (<0.8 g/kg/day) are not recommended in patients with stage 1-3 CKD [11]. However, a high protein diet may accelerate renal function decline in patients with diabetes because they increase albuminuria due to hyperfiltration which is a well-recognised mechanism of kidney damage [3]. High protein is also a source of metabolic waste products, phosphate and potassium. High protein intake (>1.3 gm/kg/day or >20% of daily calories from protein) in adults with CKD are associated with increased albuminuria, increased risk of disease progression and CV mortality and therefore should be avoided [5]. IDF guideline 2014, has advised limiting protein intake to 1 gm/kg/day if proteinuric [28]. For patients with ESKD on dialysis, higher levels of dietary protein intake should be considered due to losses that occur during dialysis [10]. Avoidance of malnutrition is important as it has adverse effect on health and CKD.
8.4. Albuminuria Control
- Albuminuria is regarded as an independent risk factor for worsening DKD; it is also an established marker of increased CV events. Therefore, any measure that reduces albuminuria will slow the progression of DKD, reduce CV complications and improve quality of life. Systematic reviews and meta-analysis reveal that ACEIs/ARBs delay progression from microalbuminuria to macroalbuminuria and can cause microalbuminuria to regress to no albuminuria in people with diabetes [50, 51]. This effect appeared to be present in patients with or without hypertension, patients with T1D or T2D, and patients with or without normal GFR. The ACEIs/ARBs dilate the efferent renal arterioles, lower intraglomerular pressure and reduce albuminuria independent of systemic BP lowering effects. Studies do not show benefit of intervention by ACE/ARB prior to onset of microalbuminuria. ACEIs/ARBs are not safe for use in pregnant subjects. Combinations of ACEIs and ARBs have been shown to provide additional lowering of albuminuria. However, they are associated with increased risk for hyperkalaemia, hypotension and AKI; therefore, combination therapy is not recommended [52]. In the treatment of the non-pregnant diabetic patient with urinary albumin excretion of 30 mg or more/day, either ACEIs or ARBs should be used unless contraindicated, irrespective of BP level [11]. Halving the amount of albuminuria with ACEIs/ARBs, results in a nearly 50% reduction in long-term risk of progression to ESKD [15]. In 2012, NKF has recommended ACEIs/ARBs in normotensive diabetic patients with albuminuria (≥ 30 mg/day) only who are at high risk of DKD and its progression (that include patients with increasing levels of albuminuria, declining GFR, increasing BP, or presence of retinopathy) [9]. In their guidelines, NKF stated that there is a lack of long-term studies to show a beneficial effect of treatment with ACEI/ARB on CKD progression in normotensive patients with diabetes and albuminuria (≥ 30 mg/day) [9]. Most people with diabetes and albuminuria have hypertension [9].
8.5. Life Style Modification
- Reduced physical activity and obesity are associated with increased incidence of diabetes, hypertension, dyslipidemia and reduced quality of life with increased morbidity and mortality. Physical activity improves quality of life, BP, lipid profiles, insulin sensitivity, and CV outcomes in patients with diabetes. Observational studies suggest that obesity is an independent risk factor for CKD [53, 54]. A systematic review of clinical trials revealed that weight loss interventions were associated with decreased albuminuria and CKD progression [55]. Therefore, weight reduction has been recommended in obese diabetic patients to prevent the progression of DKD. People with early stage DKD should be encouraged to undertake physical activity compatible with CV health and tolerance. KDIGO 2012 recommends exercise for at least 30 minutes 5 times per week [5]. Regular exercise and achievement of a healthy weight (body mass index or BMI 20-25 kg/m2 according to country-specific demographics) is recommended for patients with DKD [5]. An initiation of low intensity physical activity with gradual and slow progression should be recommended for sedentary people with diabetes unaccustomed to exercise.
8.6. Smoking Cessation
- Studies have shown that cigarette smoking is an independent risk factor for the development and progression of DKD in patients with both T1D and T2D [56-58]. Smoking is also a strong risk factor for CV morbidity and mortality in patients with diabetic as well as non-DKDs. Smoking accelerates albuminuria and decline in kidney function. Cessation of smoking reduces the progression of CKD in diabetes and also decreases the risk of CV morbidity and mortality [59]. Therefore, cessation of smoking is an important and strong recommendation in all patient with DKD [9, 28].
8.7. Treatment of Dyslipidemia
- In DKD, dyslipidemia results in increased atherosclerotic CV disease (ASCVD) that includes ischaemic heart disease, cerebrovascular disease, or peripheral arterial disease. ASCVD is an important cause of morbidity and mortality in individuals with DKD. Clinical trials have shown that lowering low density lipoprotein (LDL) cholesterol reduces the major atherosclerotic events in CKD [60]. Dyslipidemia contributes to the development and progression of DKD by causing intra-renal arteriosclerosis or direct toxicity to renal cells [61]. There is evidence to suggest that lowering lipid reduces the level of proteinuria, preserves GFR and retard the progression of renal impairment [62]. Guidelines have recommended optimum lipid control to slow the progression of nephropathy and reduce the risk of CV events [9, 27, 49, 63]. All diabetics should be encouraged to go on a life style change comprising increased physical activity, reduction in intake of saturated fat and cholesterol, as well as achievement of ideal body weight. Therapy with lipid lowering drugs, especially with statin, reduces CV morbidity and mortality in diabetics and in other patients at high risk of clinical atherosclerotic disease. In the management of dyslipidemia, ASCVD risk assessment is done based on serum lipid levels, presence or absence of established ASCVD, other risk factor identification and using an ASCVD risk calculator/ assessment tool. The absolute probability of 10-year risk of developing an ASCVD event is considered in risk stratification to help guide therapy. According to The American College of Cardiology/ American Heart Association 2017, treatment goals for dyslipidemia have been personalized according to levels of risk [63]. In persons with DM with no ASCVD, treatment efforts should target a LDL-cholesterol goal of <100 mg/dL (2.6 mmol/L), and in those with established ASCVD, an LDL-cholesterol level of <70 mg/dL (1.8 mmol/L). SIGN 2010 recommends lipid lowering drug therapy with statin for primary prevention in patients with T2D aged >40 years regardless of baseline cholesterol [11]. Patients under 40 years with type 1 or T2D and other important risk factors, e.g. microalbuminuria, should also be considered for primary prevention lipid lowering drug therapy with statin [11].It should be noted that higher doses of lipid lowering medicines especially statins are associated with increased risk of myopathy, particularly among patients with reduced kidney function [64]. Therefore, doses of lipid lowering medicines should be modified in moderate to advanced CKD (stages 3-5). Strategies by using combination therapy with low dose statins e.g. statin/ ezetimibe combination and/or a PCSK9 (proprotein convertase subtilisin-kexin type 9) inhibitor, are being emerged to treat higher lipid levels [14, 60]. NKF 2012 guideline, recommends not initiating statin therapy in patients with diabetes who are treated by dialysis because of lack of significant effect of statin therapy on the primary CV outcome in those patients [9].
8.8. Use of Aspirin/ Clopidogrel
- Microalbuminuria is an established risk for CVD in a patient with DKD as well as in the general population [65-67]. A subgroup analysis of one large trial revealed that patients with DKD might benefit from aspirin therapy [68]. Guidelines have recommended aspirin therapy as a secondary prevention strategy in those with diabetes and a history of ASCVD. The ADA 2019, in their Standards of Care, has recommended low-dose aspirin therapy as a primary prevention strategy in those with diabetes at increased CV risk after a discussion with the patient on the benefits versus increased risk of bleeding [42]. The recommendation includes most men and women with diabetes aged ≥50 years who have at least one additional major risk factor (family history of premature ASCVD, hypertension, dyslipidemia, smoking, or CKD/ albuminuria) who are not at increased risk of bleeding (e.g., older age, anemia, renal disease). Low dose aspirin has been considered by ADA 2019, in the context of high CV risk with low bleeding risk, but generally not recommended in older adults. However, other guidelines do not recommend aspirin or clopidogrel for adults having diabetes without evidence of CVD [11, 14, 27]. For patients with ASCVD and documented aspirin allergy, clopidogrel (75 mg/day) should be used. Combination of clopidogrel with aspirin should be avoided in patients with CKD unless compelling indications are present. CKD patients are at increased risk of bleeding compared with the general population. In patients aged less than 21 years, aspirin use is generally contraindicated due to the associated risk of Reye syndrome [42].
8.9. Restriction of Salt and Potassium
- In subjects with CKD having reduced eGFR, impaired excretion of sodium and potassium are often present. High sodium intake increases BP, induces glomerular hyperfiltration and thereby increases proteinuria. Lowering salt intake reduces BP as well as albuminuria, the later by lowering intraglomerular pressure. Restriction of dietary sodium may be useful to control BP and reduce CV risk. Restriction of salt intake limited to 2 gm per day of sodium (corresponding to 5 gm of salt or NaCl) unless contraindicated, has been recommended in patients with DKD [5]. Serum potassium level may increase with the use of ACEIs/ARBs or mineralocorticoid receptor antagonists (spironolactone). Serum potassium level should be maintained to a safe level. Restriction of dietary potassium may be necessary to control serum potassium level as hyperkalaemia has adverse effects on neuromuscular and heart function. Recommendations for dietary sodium and potassium intake should be individualized on the basis of comorbid conditions, medication use, BP level, and laboratory data [10].
8.10. Avoidance of Nephrotoxic Agents
- Patients with eGFR <60 mL/min/1.73m2, should avoid or minimise all nephrotoxic agents (e.g. NSAIDs, aminoglycosides, high phosphate containing drugs and iodine containing contrast dyes) [10, 15]. Potassium-sparing diuretics should be used with caution or avoided altogether. Long-term proton pump inhibitor (PPI) use should be avoided unless with clear indication [15]. Gadolinium used as a contrast medium for MRI scan should be avoided in stage 4 & 5 CKD due to development of nephrogenic systemic fibrosis weeks or months after administration [5]. Drug-induced renal impairment is generally reversible, provided the nephrotoxicity is recognized early and the offending medication is discontinued.
8.11. Urate Lowering Therapy
- Hyperuricemia with renal impairment is an indication of urate lowering therapy [69]. There are studies suggesting that in patients with T2D with hyperuricemia, eGFR significantly improves when serum urate levels are reduced to normal (<6 mg/dL) by uric acid lowering drugs (e.g. allopurinol, febuxostat) [70-73]. Therefore, uric acid reduction is a potential strategy to delay CKD progression. Hyperuricemia is also an established component of metabolic syndrome. However, a recent study in Japanese population, demonstrated that febuxostat had no effect on renal function in asymptomatic hyperuricemic patients with stage 3 CKD [74]. In addition to causing musculoskeletal disease, chronic hyperuricemia may be complicated by renal stone formation and, if severe, renal impairment due to the development of interstitial nephritis as a result of urate deposition in the kidney. [69]. A therapeutic target of 360 μmol/L (6 mg/dL) is recommended by the British Society of Rheumatology guidelines [75].
8.12. Screening for Complications
- When the eGFR is <60 mL/min/1.73m2, screening and management for potential complications of CKD is indicated [10]. These include anemia, mineral bone disease, secondary hyperparathyroidism, acidosis, electrolyte imbalance and hyperuricemia. Renal complications of DKD include increased risk of urinary tract infections, which may be further increased with the use of SGLT2 inhibitors. Type 4 (hyperkalaemic, low–anion gap) renal tubular acidosis is more common in patients with T2D, especially those with moderate renal insufficiency [76].
8.13. Early Vaccination
- People with diabetes and CKD are often immunocompromised; they are at higher risk for hepatitis B infection and are more likely to develop complications from influenza and pneumococcal infections. Early vaccination against hepatitis B virus should be given in patients likely to progress to ESKD and require RRT (dialysis or kidney transplantation) [5, 77]. Annual influenza vaccination, unless contraindicated is recommended in all adults with CKD [5]. They should also receive pneumococcal vaccine [77]. Prophylactic vaccinations against influenza viruses and pneumococci have proved benefits in people with dialysis.
8.14. Regular Monitoring
- Serum creatinine, potassium levels and urinary ACR should be done periodically to monitor the response to treatment with ACEIs/ARBs as well as to see the progression of CKD [10]. People with diabetes are at higher risk of AKI than those without diabetes. In particular, many antihypertensive and oral antidiabetic medications (e.g., diuretics, ACEIs/ARBs, and SGLT2 inhibitors can reduce intravascular volume, renal blood flow, and/or glomerular filtration. Acute kidney injury is usually diagnosed by a rapid increase in serum creatinine, which is also reflected as a rapid decrease in eGFR over a relatively short period of time.
8.15. Referral to Nephrologist
- Patients having kidney disease in DM, referral to a nephrologist should be considered for (i) uncertainty about the etiology of kidney disease (atypical presentation, patients with heavy proteinuria, microscopic or macroscopic haematuria and cellular casts in the urine), (ii) difficult management issues (deterioration of kidney function, resistant hypertension, volume overload, complications of CKD e.g. anemia, mineral bone disease, secondary hyperparathyroidism, acid-base imbalance and electrolyte disturbances), or (iii) advanced kidney disease (stage 4/5) requiring management and evaluation for RRT (dialysis or kidney transplantation). Diabetes is also a known risk factor for arteriovenous fistula maturation failure. All patients with stage 4 CKD, dialysis access planning is vitally important [52].
9. Discussion
- Diabetes mellitus is a major worldwide health problem and DKD is one of the most important complication of both type 1 and T2D. Analysis of data from the US national representative data system revealed that among the diabetes-related complications there was the smallest decline in ESRD as compared with acute myocardial infarction, hyperglycemic crisis, stroke and lower-extremity amputations [78]. The decline of most of the diabetes-related complications may reflect a combination of advances in clinical care, enhanced management of risk factors, and improvement of health promotion efforts directed at patients with diabetes. Currently, there are substantial numbers of guidelines around the world to manage DKD at the national and international levels. In the various guidelines there are certain controversies and differences in diagnosis and management of DKD [7, 8]. However, all the guidelines concur with the basic recommendations in the management of DKD. Some controversies in the diagnosis and management of DKD are summarized in Table 1 and Table 2 respectively.
|
|
10. Conclusions
- DKD usually progresses to ESKD; it is also a risk for CV morbidity and mortality. Early diagnosis and management with multifactorial interventions is important to reduce the progression of early DKD to ESKD and also to reduce the risk of CV events. Among the antidiabetic agents, SGLT2 inhibitors (dapagliflozin, canagliflozin, and empagliflozin) and GLP-1 receptor agonists (liraglutide and semaglutide) have been reported to improve renal outcomes by reducing the amount of albuminuria and preventing GFR loss in patients with T2D. The key recommendations in the management of early stage DKD can be summarized as follows:• Annual screening for microalbuminuria & renal function by eGFR • Optimum glycemic control (patient centred approach; target HbA1c ~7%) • Adequate BP control (patient centred approach) • Limitation of excess dietary protein for people with nondialysis-dependent DKD • Control of albuminuria (if ≥30 mg/day) by using ACEIs/ARBs unless contraindicated • Exercise & maintenance of ideal body weight• Cessation of smoking • Control of dyslipidemia • Use of aspirin/ clopidogrel • Dietary salt restriction• Avoidance of nephrotoxic agents• Screening for other complications of DKD when eGFR <60 mL/min/1.73m2• Early vaccination• Timely nephrology referral
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML