-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2019; 9(3): 96-103
doi:10.5923/j.ajmms.20190903.06

Assessment of Bacteriological Quality of Borehole Water, Sachet Water and Well Water in Bingham University Community
Ajobiewe H. F.1, Ajobiewe J. O.1, 2, Mbagwu T. T.1, Ale T.2, Taimako G. A.1
1Biological Science Department Bingham University, Karu, Nasarawa State, Nigeria
2Microbiology Department National Hospital Abuja FCT, Nigeria
Correspondence to: Ajobiewe H. F., Biological Science Department Bingham University, Karu, Nasarawa State, Nigeria.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This study was conducted to investigate the portability of 20 water samples collected for two weeks from boreholes, wells and sachet water consumed within Bingham University community. The objective was to determine the bacteriological quality of these water samples. Results obtained after carrying out bacteriological analysis showed the presence of organisms in all the water samples. Organisms isolated include Escherichia coli, Klebsiella pneumoniae, Salmonella spp., Pseudomonas aeruginosa. Klebsiella pneumoniae having the highest percentage occurrence of 40% for the first week and 30% for the second week, Escherichia coli having the highest percentage occurrence of 50% for the second week and 30% for the first week, Salmonella spp. with 30% and 20% for the first and second week respectively and Pseudomonas aeruginosa with percentage occurrence of 10% for the second week. The highest bacteria count was obtained in the well water samples and the least was from sachet water. Findings showed that water consumed within Bingham University community is not completely suitable for consumption and should be treated properly. There is a significant correlation between bacterial infection and different water samples.
Keywords: Bacteriological, Salmonella spp., Escherichia coli, Pseudomonas spp., Klebsiella spp., Water
Cite this paper: Ajobiewe H. F., Ajobiewe J. O., Mbagwu T. T., Ale T., Taimako G. A., Assessment of Bacteriological Quality of Borehole Water, Sachet Water and Well Water in Bingham University Community, American Journal of Medicine and Medical Sciences, Vol. 9 No. 3, 2019, pp. 96-103. doi: 10.5923/j.ajmms.20190903.06.
Article Outline
1. Study Background/Literature Reviews
- Water is a universal solvent, which consist of hydrogen and oxygen atoms. Chemically, it could be defined as a chemical substance with two atoms of hydrogen and one atom of oxygen in each of its molecules; hence the molecular formula is H2O [1]. It is formed by the direct reaction of hydrogen with oxygen;
 Water is essential to sustain life, and a satisfactory supply must be made available to consumers. It is a critical requirement in the maintenance of metabolic functions and homeostasis (the ability to maintain stable body conditions) in living cells. The human body is composed of about 60% water by weight in adult males, 50% in females and 70% in new born infants [2]. Portable water is water pure enough to be consumed or used with low risk of immediate or long term harm. Water is one of the most important necessities to all forms of life on this planet [3]. Therefore, adequate and safe water supply should be available to humans, plants, and animals in all parts of the world. The regular intake of adequate amounts of water is essential in the maintenance of good health and well-being. The approximate human dietary requirement of water is estimated to be two litres per day for an average adult [4]. According to the World Health Organization every effort should be made to achieve a safe drinking water supply in every community of the world because it is known that improving access to safe drinking-water can result in significant benefits to health [5]. The most important attribute of drinking water that has to be assured and maintained is its safety and quality for human consumption [6]. Drinking water must be free of harmful contaminants, such as pathogenic microorganisms, toxic substances, physical and chemical residues, undesirable organoleptic properties like odour, colour, and taste [7].The issue of access to potable water is very important in Nigeria, where 48% of Nigerians depend on surface water for domestic use, 57% use hand dug wells, 20% harvest rain, 14% have access to pipe borne water, and 14% have access to borehole water sources (FGN, 2007). The quality and quantity of pipe borne water for drinking is deteriorating in the country due to inadequacy of treatment plants, direct discharge of untreated sewage into rivers and streams, and inefficient management of piped water distribution system [9].It has been estimated that the mortality of water associated diseases exceeds 5 million people per year around the world [10]. Of these, there are reports that more than 50% of these deaths are associated with microbial intestinal infections, particularly with cholera and typhoid. Microorganisms of concern in contaminated water include bacterial agents of diarrhoea and gastroenteritis namely Salmonella spp., Shigella spp., Escherichia coli and Vibrio cholera) [11]. Protozoal agents of diarrhoea include Entamoeba histolytica, Giardia lamblia, Balantidium coli [12]. Presence of faecal coliforms or Escherichia coli is used as an indicator for the presence of any of these water borne pathogens [13]. It is recommended that good quality water should be colourless, odourless, tasteless, and free of faecal contamination and chemicals in harmful amounts [11].Based on research on the prevalence of common water borne diseases in some parts of Nigeria, between the year 2002-2008, typhoid cases ranked highest among the water related diseases, followed by cholera, hepatitis and Dracunculiasis [13]. In Sokoto, the cases of diarrhoea and dysentery in children between the ages of 5 and below was of high proportion in the year 2004 and 2005 than in adults [14]. This incidence of waterborne diseases is as a result of inability to gain access to portable drinking water most especially people living in the rural areas of the country. People search for drinking water from all sorts of unsafe water sources, which expose them to all kinds of dangers related to drinking of unsafe water [13]. Microbiological quality assessment is of principal concern because of the acute risk to health posed by viruses, bacteria and helminths in drinking-water. Therefore, assessment of drinking-water is primarily a health-based activity which emphasizes the protection of public health through ensuring that the water supplied is of a good quality. Generally, borehole water is considered to have better microbial quality than that of hand dug well water because borehole water is from deep aquifer while hand dug well water is from shallow aquifers which makes it more susceptible to microbial pollution [15].
Water is essential to sustain life, and a satisfactory supply must be made available to consumers. It is a critical requirement in the maintenance of metabolic functions and homeostasis (the ability to maintain stable body conditions) in living cells. The human body is composed of about 60% water by weight in adult males, 50% in females and 70% in new born infants [2]. Portable water is water pure enough to be consumed or used with low risk of immediate or long term harm. Water is one of the most important necessities to all forms of life on this planet [3]. Therefore, adequate and safe water supply should be available to humans, plants, and animals in all parts of the world. The regular intake of adequate amounts of water is essential in the maintenance of good health and well-being. The approximate human dietary requirement of water is estimated to be two litres per day for an average adult [4]. According to the World Health Organization every effort should be made to achieve a safe drinking water supply in every community of the world because it is known that improving access to safe drinking-water can result in significant benefits to health [5]. The most important attribute of drinking water that has to be assured and maintained is its safety and quality for human consumption [6]. Drinking water must be free of harmful contaminants, such as pathogenic microorganisms, toxic substances, physical and chemical residues, undesirable organoleptic properties like odour, colour, and taste [7].The issue of access to potable water is very important in Nigeria, where 48% of Nigerians depend on surface water for domestic use, 57% use hand dug wells, 20% harvest rain, 14% have access to pipe borne water, and 14% have access to borehole water sources (FGN, 2007). The quality and quantity of pipe borne water for drinking is deteriorating in the country due to inadequacy of treatment plants, direct discharge of untreated sewage into rivers and streams, and inefficient management of piped water distribution system [9].It has been estimated that the mortality of water associated diseases exceeds 5 million people per year around the world [10]. Of these, there are reports that more than 50% of these deaths are associated with microbial intestinal infections, particularly with cholera and typhoid. Microorganisms of concern in contaminated water include bacterial agents of diarrhoea and gastroenteritis namely Salmonella spp., Shigella spp., Escherichia coli and Vibrio cholera) [11]. Protozoal agents of diarrhoea include Entamoeba histolytica, Giardia lamblia, Balantidium coli [12]. Presence of faecal coliforms or Escherichia coli is used as an indicator for the presence of any of these water borne pathogens [13]. It is recommended that good quality water should be colourless, odourless, tasteless, and free of faecal contamination and chemicals in harmful amounts [11].Based on research on the prevalence of common water borne diseases in some parts of Nigeria, between the year 2002-2008, typhoid cases ranked highest among the water related diseases, followed by cholera, hepatitis and Dracunculiasis [13]. In Sokoto, the cases of diarrhoea and dysentery in children between the ages of 5 and below was of high proportion in the year 2004 and 2005 than in adults [14]. This incidence of waterborne diseases is as a result of inability to gain access to portable drinking water most especially people living in the rural areas of the country. People search for drinking water from all sorts of unsafe water sources, which expose them to all kinds of dangers related to drinking of unsafe water [13]. Microbiological quality assessment is of principal concern because of the acute risk to health posed by viruses, bacteria and helminths in drinking-water. Therefore, assessment of drinking-water is primarily a health-based activity which emphasizes the protection of public health through ensuring that the water supplied is of a good quality. Generally, borehole water is considered to have better microbial quality than that of hand dug well water because borehole water is from deep aquifer while hand dug well water is from shallow aquifers which makes it more susceptible to microbial pollution [15].2. Literature Review
- Water is essential for living things, both in the composition of their cells and in the environment surrounding them. Organisms are made up of between 60 and 95 per cent water by weight, and even inert, dormant forms like spores and seeds have a significant water component. This dependence on water is a function of its unique properties, which in turn derive from its polar nature [15].To humans, water is indispensable. In the home, water is used for cooking, washing, bathing and other domestic uses. Industrially, water is the starting point of most processes. The chemist says that water is a universal solvent following his findings that most chemicals are soluble in water. For the biologist, it is even more important for the growth of organisms and for carrying out fermentation for the production of products useful to man [16].An examination of water quality is basically a determination of the organisms, minerals and organic compounds contained in the water. The basic requirements for drinking water are that it should be free from pathogenic organisms, contain no compounds that have adverse effects on human health, be fairly clear, be non-saline, contain no compounds that cause an offensive taste or smell and cause no corrosion [17].Water treatment employs aeration, coagulation and flocculation, sedimentation, slow sand filtration and rapid filtration and disinfection. The principal methods of purifying water on a small scale are those used locally in areas where water harvesting is carried out include sedimentation, coagulation, boiling and filtration. Boiling is the most satisfactory way of destroying disease-producing organisms in water. It is equally effective whether the water is clear or cloudy, whether it is relatively pure or heavily contaminated with organic matter. Boiling destroys all forms of disease producing organisms usually encountered in water whether they are bacteria, viruses, spores, cysts or ova [18].MAIN SOURCES OF DOMESTIC WATERThe major sources of drinking water include: Streams, Lakes, Rivers, Ponds, Rainwater and Underground water (spring, wells, and boreholes). Underground water is safer and purer for domestic use than surface water because the ground itself serves as an effective filter medium [19]. Water from deep wells and deep springs usually dissolves a lot of salts and other minerals which is a major problem with underground water and so the water becomes salty, sometime too salty or "hard" for any use unless the salts are removed which is expensive [20].SOURCES OF WATER POLLUTIONThe main sources of boreholes and well water pollutants are industrial, domestic and agricultural waste. Irrigation water and runoff water from rain carrying fertilizers, herbicides, feacal matters mix with natural water bodies and pollute the water [21]. Recycling of treated/inadequately treated waste water by mixing them with natural water bodies adds microorganisms. When septic tanks are built near the water bodies mixing or seeping of excreta may occur and this may act as a source of waterborne pathogens. Waste water from abattoirs and animal processing plants also contribute to the water borne pathogens. Droppings from nearby birds and faecal materials of domestic and wild animals including those of diseased ones are another potential source [22].BACTERIOLOGICAL WATER ANALYSISBacteriological water analysis is a method of analysing water to estimate the numbers of bacteria present and, if needed, to find out what sort of bacteria they are. It represents one aspect of water quality. It is an analytical procedure which uses samples of water and from these samples determines the concentration of bacteria. It is then possible to draw inferences about the suitability of the water for use from these concentrations [23]. The pathogenic organisms which may be present in water are numerous and identifying these organisms individually in practice (bacteria, protozoa, helminths etc.) is difficult. As their presence is always linked to faecal pollution (except for guinea worm), it is preferable to look for organisms which are “indicators” of this pollution [22]. The common feature of all these routine screening procedures is that the primary analysis is for indicator organisms rather than the pathogens that might cause concern [16]. Indicator organisms are bacteria such as non-specific coliforms, Escherichia coli and Faecal Streptococci such as Enterococcus faecalis that are very commonly found in the human or animal gut and which, if detected, may suggest the presence of sewage [24]. It is therefore reasonable to summarize that if indicator organism levels are low, then pathogen levels will be very much lower or absent. The count of those colonies which develop with a characteristic appearance gives the number of faecal coliforms in the sample of water [20]. Judgments as to suitability of water for use are based on extensive precedents and relate to the probability of any sample population of bacteria being able to be infective at a reasonable statistical level of confidence. Analysis is usually performed using culture, biochemical and sometimes optical methods. When indicator organism’s levels exceed pre-set triggers, specific analysis for pathogens may then be undertaken and these can be quickly detected (when suspected) using specific culture methods or molecular biology [25].PORTABLE WATERPortable water is free of pathogens and toxic chemicals. Purification can be done by coagulation, which is by adding alum, Nitrogen aluminates or Ferric chloride to the water. Using the sand bed method, filtration can be carried out or repair sand bed filters can also be used. For the correction of pH of portable water, limestone is added. Portable water is often treated by chlorination. This makes the water free from any coliform organism no matter how polluted the original water may have' been [26]. Water treatment involves the conversion of water taken from the natural sources, the “raw water” into that suitable for domestic use. Ground water and surface water usually require more critical treatment than rain water. Harvested water also requires some form of treatment. Most important is the removal of pathogenic organisms and toxic substances such as heavy metals that can cause health problems [27]. Storage of water may be regarded as a form of treatment. Schistosoma mansoni cercariae are normally unable to survive 48 hours of storage. Also the number of faecal Escherichia coli will be considerably reduced when the raw water is subjected to storages [29].NON PORTABLE WATERNon- potable water is one contaminated with domestic and industrial waste. There are so many characteristics that make water not potable such as taste, smell, pH, colour/turbidity and mineral salts [28].MICROBIOLOGICALLY CONTAMINATED WATERWater may contain numerous pathogenic organisms and thereby become a means of transmission for many diseases. These includes: Typhoid and paratyphoid fever, Hepatitis A, Cholera, Poliomyelitis, Diarrhoea (caused by Escherichia coli, Salmonellae, Yersinia enterocolitica), Viral gastroenteritis, Bacillary dysentery (caused by various species of Shigella), Campylobacter dysentery, Amoebic dysentery, Giardia (lambliasis), Balantidiasis, Helminthiasis cause by Ascaris and Trichuris [29]. Besides these diseases, water is also involved in the transmission of “water- based” diseases (that is, diseases of which the causative agent passes part of its life cycle in an aquatic plant or animal): The different schistosomiasis or bilharziasis: diseases caused by helminths (worms) which are usually contracted by contact with infected water but sometimes also via the oral route. Dracunculiasis (Guinea worm), transmitted only by drinking infested water [30]. Lastly, water may also transmit: Leptospirosis: a bacterial disease which is contracted primarily by contact with water contaminated with the infected urine of various animals (principally the rat). All the infectious diseases transmitted by water with exception of guinea worm are linked to the pollution of the water by the excreta of humans or other animals infected. One last category of water related diseases is those with an insect vector which develops in or lives near to the water, for example malaria, dengue and yellow fevers, and onchocerciasis [30].WATER BORNE DISEASESWater-borne diseases are any illness caused by water people drink that is contaminated by animal or human faeces, which contain pathogenic microorganisms. Waterborne diseases are caused by pathogenic microorganisms that most commonly are transmitted in contaminated fresh water. Water should be harmless to health and have an appearance and taste acceptable to the population. Ideally the water supplied should meet the quality standard of the WHO. Quite a number of human pathogens find their way into a susceptible host through contaminated water. These pathogens often called waterborne pathogens, have the ability to survive at least for a short period in water and thus water may act as a route of transmission for them [30]. Waterborne diseases are posing a serious threat to health since the potential of contaminated water to transmit disease is very high. Often they lead to epidemic. According to a WHO survey about 30,000 people die from water-related diseases every day [24]. About 80% of all illness in developing countries is water related [24]. Typhoid fever: this is caused by ingestion of Salmonella typhi bacteria in food or water. Infection causes a sudden high fever, nausea, severe headache, and loss of appetite. It is sometimes accompanied by constipation or diarrhoea [30]. Hepatitis (A & E): This is caused by viral infection. Symptoms include yellowing of the skin and eyes (jaundice), dark urine, fatigue, nausea and vomiting. Two forms of the disease, hepatitis A and E, are primarily caused by ingestion of faecally contaminated drinking water [31]. Hepatitis A causes about 1.5 million infections each year (mostly in children), and can occur in epidemics. Hepatitis E is less common than hepatitis A, and occurs mainly in epidemics caused by monsoon rains, heavy flooding, contamination of well water, or massive uptake of untreated sewage into water bodies. No specific treatment exists for hepatitis A or E, but most (>98%) patients recover completely. Hepatitis can have more serious effects on older or immunocompromised people, and pregnant women are particularly vulnerable to hepatitis E, with approximately 20% mortality rates [32]. Haemorrhagic colitis and haemolytic uremic syndrome: This is an infection associated with Escherichia coli 0157:H7. The latter occur particularly in children [33]. Other water borne diseases are Polio [31], Legionellosis [32], Leptospirosis [34], Diabetes [35], Myocarditis [36], Guillain-Barre syndrome [37] Gastric cancer [38], Reactive arthritis [39] and so on.Water-Washed Diseases: Water-washed diseases are diseases caused by inadequate use of water for domestic and personal hygiene. Control of water-washed diseases depends more on the quantity of water than the quality. Most of the diarrhoeal diseases should be considered to be water-washed as well as water-borne. Four types of water-washed diseases are; soil-transmitted helminths, acute respiratory infections (ARI), skin and eye diseases, and diseases caused by fleas, lice, mites or tick [31]. WATER QUALITY: The importance of high quality water cannot be over-emphasized as it sustains human life and maintains health. Most waters, before they reach the consumer, have been exposed to greater or lesser amount of contamination, but in the majority of case, they have also undergone a more or less complete purification by natural agencies [39].
3. Methods
- SAMPLING SITESWater samples were collected from boreholes, wells and sachet water within Bingham community. The water sources were labelled as: Sample A1 and A2
 Girls HostelSample B1 and B2
Girls HostelSample B1 and B2 Boys HostelSample C1 and C2
Boys HostelSample C1 and C2 Staff quartersSample D1 and D2
Staff quartersSample D1 and D2 Cafeteria and Administrative blockSample E1 and E2
Cafeteria and Administrative blockSample E1 and E2 Sachet waterSample F1 and F2
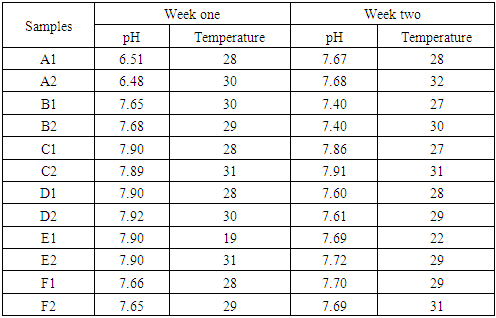
Sachet waterSample F1 and F2 Well waterSamples A- D representing the water samples from borehole source.The numbers 1 and 2 indicating morning and evening samples respectively.SAMPLE COLLECTION Water samples were aseptically collected in sterilized bottles from various sources within Bingham university community. Samples were collected from boreholes, well, sachet water. All samples were transported to the laboratory and analysis was carried out within two hours.PHYSIOCHEMICAL ANALYSIS: In the pH determination a glass electrode meter was used. The pH meter was standardized with buffer solution of pH 4 for acidic, 7 for neutral and 9 for alkaline. The pH scale was read when the valves were stabilized within few minutes. The meter was cleaned with tissue paper at intervals after rinsing with distilled water. At the point of sample collection, a mercury thermometer calibrated in centigrade was immersed in the water samples. The values obtained were recorded in degree centigrade. Normal aseptic procedures in microbiology laboratory were strictly adhered to during bacteriological analysis of the water samples following the method of Chessbrough, (2000) [20].
Well waterSamples A- D representing the water samples from borehole source.The numbers 1 and 2 indicating morning and evening samples respectively.SAMPLE COLLECTION Water samples were aseptically collected in sterilized bottles from various sources within Bingham university community. Samples were collected from boreholes, well, sachet water. All samples were transported to the laboratory and analysis was carried out within two hours.PHYSIOCHEMICAL ANALYSIS: In the pH determination a glass electrode meter was used. The pH meter was standardized with buffer solution of pH 4 for acidic, 7 for neutral and 9 for alkaline. The pH scale was read when the valves were stabilized within few minutes. The meter was cleaned with tissue paper at intervals after rinsing with distilled water. At the point of sample collection, a mercury thermometer calibrated in centigrade was immersed in the water samples. The values obtained were recorded in degree centigrade. Normal aseptic procedures in microbiology laboratory were strictly adhered to during bacteriological analysis of the water samples following the method of Chessbrough, (2000) [20].4. Results
|
|
|
|
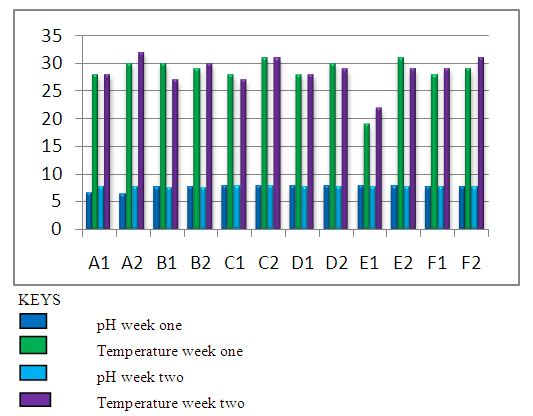 | Figure 2. Graphical Representation of Physicochemical Analysis |
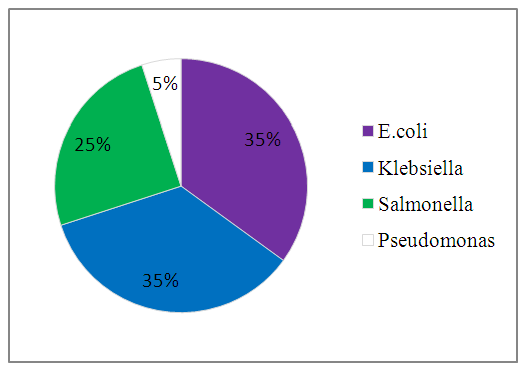 | Figure 3. Percentage Occurrence |
5. Discussion
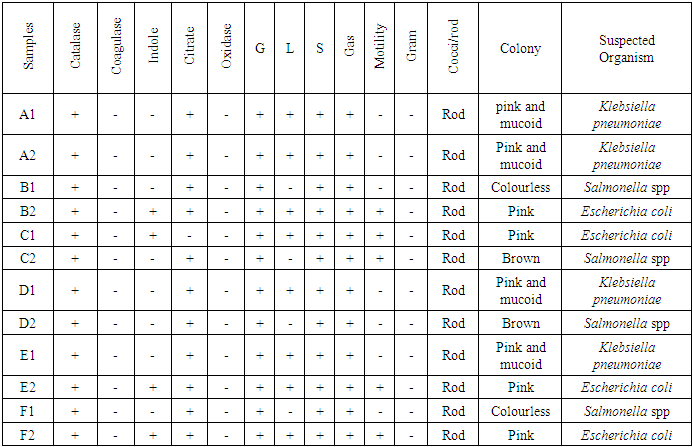
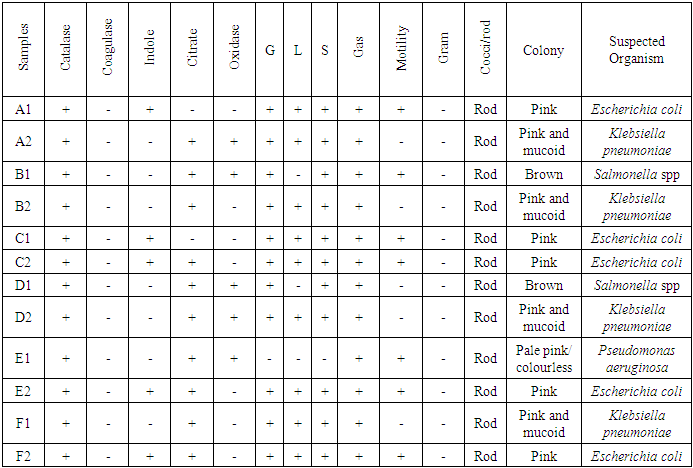
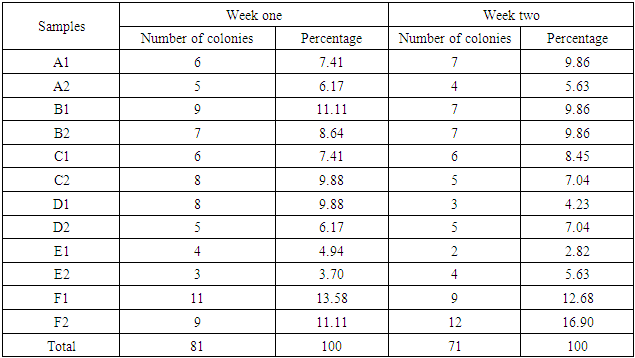
- From the results obtained during microbiological analysis, it was observed that the bacteria count of sachet water consumed within Bingham University have a lower count compared to the borehole and well water. Based on the analysis, it can be said that the sachet water is a better source of drinking water when compared with other sources. The organisms Escherichia coli, Klebsiella pneumoniae, Salmonella spp, Pseudomonas aeruginosa were isolated from the samples. The findings is confirmed with the work done by [55] in which these organisms were isolated in addition to other organisms. From table 3.4 above, it was observed that water from well has the highest bacterial load which agrees with the work of [55] [56]. 13.58% of Salmonella spp and 11.11% of Escherichia coli from the first source and 12% of Klebsiella pneumoniae and 16% of Escherichia coli from the second source when compared with other sources. The sachet water has the least with 4.94% of Klebsiella pneumoniae and 3.7% of Escherichia coli for the first week and 2.82% of Pseudomonas aeruginosa and 5.63% of Escherichia coli for the second week. Borehole water which is represented based on the four site of collection had Escherichia coli and Klebsiella pneumoniae with the highest percentage of occurrence in line with [57] when compared with Salmonella. Based on the count, 7.41% and 6.17% of Klebsiella pneumoniae was obtained from the girls hostel borehole, 11.11% of Salmonella and 8.64% of Escherichia coli from the boys hostel borehole, 7.41% of Escherichia coli and 9.88% of Salmonella from the staff quarters, 9.88% of Klebsiella pneumoniae and 9.88% of Salmonella from the administrative area for the first week. For the second week, 9.86% of Escherichia coli and 5.63% of Klebsiella pneumoniae was obtained from the girls hostel, 9.86% of Salmonella and 9.86% of Klebsiella pneumoniae from the boys hostel, 8.45% and 7.04% of Escherichia coli from staff house, 4.23% of Salmonella and 7.04% of Klebsiella pneumoniae from the cafeteria. From the general observations in table 3.4 above, the most predominant organism in borehole and sachet water is Escherichia coli. This finding is not relating with the findings of [58] [59] that had Escherichia coli free water samples from borehole and sachet water. Klebsiella pneumoniae was next then Salmonella and the least Pseudomonas aeruginosa. This order of occurrence correlates with the work of [60].Using the t-test statistic, T-cal = 5.965 for the first week which is higher than T-tab 0.05 = 2.571 and T-tab 0.01 = 4.032, therefore, alternate hypothesis – there is a significant difference is accepted. For the second week, T-cal = 3.841 which is greater than T-tab at 0.05 = 2.571 and less than T-tab 0.01 = 4.032, that is, there is a significant difference at 0.05 but no significant difference at 0.01. The pH is the negative logarithm to base 10 of molar concentration, measured in units of moles per liter, of hydrogen ions. It expresses the acidity or alkalinity of a solution on a logarithm scale on which 7 is neutral, lower values are more acid and higher value are more alkaline. From the physiochemical results obtained during this work, the pH obtained from different water sources ranges from 6.48 – 7.90 with a mean value of 7.59 and 7.40 – 7.91 with a mean value of 7.66 for the first and second week respectively. The mean value for the first week correlate with that of [50] who obtained a mean range of 7.2 – 7.5 and the mean values obtained from both weeks are greater than that of [51]. The values recorded above indicated that the water consumed with Bingham University community is within the WHO standard for drinking water which is 6.5 – 8.5 [43]. Microbial activities of aquatic microorganisms are greatly affected by temperature. The mean temperature acquired from the first and second week, that is, 28.24 and 28.58 respectively is a suitable temperature for the growth of mesophilic organism which grow within the range of 25°C to 40°C [24]. The temperature of water is also affected by weather condition [52]. This explains the reason for the rise in temperature of samples collected in the evening and the fall in temperature of samples collected in the morning after rainfall. Samples E1 from both weeks have the least temperature values and this is because both samples are refrigerated water. The mean temperatures for both weeks are in line with EPA standard temperature for drinking water which is < 40°C [25].
6. Conclusions
- It was observed that the microbial indicators Escherichia coli and Klebsiella pneumoniae populate most of the water samples. The coliform counts of all the water samples were generally high exceeding the WHO standard of zero coliform count per 100ml [5]. Therefore, Bingham University water sources are polluted with feacal contamination and the water is not fit for consumption before appropriate treatment. Bingham university sachet water can be considered as the best source of drinking water because the bacteria load is the lowest. Water suitable for consumption should be free from diseases producing organisms or large number of non-pathogenic organisms [53].
7. Recommendations
- Awareness of the community towards the dangers associated with the use of contaminated water should be increased and also the dangers of constructing soak- aways near a water source. Sachet water sold within Bingham University should be adequately treated before use and NAFDAC should enforce and ensure strict compliance to the standards as regard the production and sales of packaged water. Well water should be boiled before consumption to ensure good health safety.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML