-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2019; 9(2): 54-57
doi:10.5923/j.ajmms.20190902.04

Influence of Prenatal Hyperandrogenization on the Condition of Indicators of the Endocrine Profile in Rats
Khamid Yakubovich Karimov, Feruza Tairovna Maksudova
Department of Molecular Medicine and Cell Technologies, Scientific Research Institute of Hematology an Blood Transfusion of the Ministry of Health of the Republic of Uzbekistan, Tashkent, Uzbekistan
Correspondence to: Feruza Tairovna Maksudova, Department of Molecular Medicine and Cell Technologies, Scientific Research Institute of Hematology an Blood Transfusion of the Ministry of Health of the Republic of Uzbekistan, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The aim of the study was to evaluate the effect of prenatal hyperandrogenism on the endocrine profile indicators in rats fed different levels of calories. Studies were performed on 90 outbred white pregnant rats fed on a high-calorie diet (HCD) and rational diet (RD). Concentrations of prolactin (PRL), luteinizing hormone (LH), follicle-stimulating hormone (FSH), estradiol (Est), total testosterone (TTS), cortisol (Cort) and dehydroepiandosterone sulfate (DHEA-s) were measured. Results showed that prenatal androgenization caused an imbalance of the endocrine profile in rats, which is more pronounced with a high-calorie diet.
Keywords: Hyperandrogenism, High-calorie diet (HCD), Endocrine profile, Pregnant rats
Cite this paper: Khamid Yakubovich Karimov, Feruza Tairovna Maksudova, Influence of Prenatal Hyperandrogenization on the Condition of Indicators of the Endocrine Profile in Rats, American Journal of Medicine and Medical Sciences, Vol. 9 No. 2, 2019, pp. 54-57. doi: 10.5923/j.ajmms.20190902.04.
Article Outline
1. Introduction
- Currently, the increasing interest of scientists all over the world is attracted to the study of one of the important problems of medicine - the study of the reproductive health of women [1, 2]. The urgency of the problem is emphasized by the increase in recent years in the frequency of various pathologies of the reproductive system in women, developing under the influence of various exogenous and endogenous factors [4]. Today, one of the most frequent causes of impaired fertility in women [8, 10], i.e. infertility, are disorders of the mechanisms of the endocrine profile, in particular polycystic ovary syndrome (PCOS, Stein-Leventhal syndrome) [5, 6, 7].Taking into account the fact that PCOS is a multiendocrine pathology, research is being actively conducted to study the mechanisms of formation of the syndrome involving the hypothalamic-pituitary system, adrenal glands and ovaries [9, 11, 12]. Available publications of the results of studies on the pathogenetic aspects of the syndrome emphasize that hyperandrogenism is one of the important links in its development. There are also opinions that already in the prenatal period, prolonged exposure of the androgens to the fetus may lead to the formation of this type of steroidogenesis in the ovary, in which PCOS may later occur [3].According to statistics, pathology is often combined with obesity. In the period of postnatal development, obesity acts as an additional factor leading to the aggravation of PCOS and gross hormonal disorders. High-calorie diet (HCD) with this pathology leads to the development of complications and significantly affects the quality of life of patients with PCOS.The presence of certain restrictions in conducting scientific experiments with the participation of people, to study the mechanisms of PCOS formation, which allow a better understanding of the mechanism of development of pathology, determine the need for their implementation in laboratory animals [13].
2. Main Body
2.1. Purpose of the Study
- Study of the effect of prenatal hyperandrogenism on the state of endocrine profile indicators in the experiment.
2.2. Material and Methods of Investigation
- Studies conducted on 90 mongrel white pregnant rats that are on a normal laboratory diet. All animal procedures were carried out in accordance with ethical rules. On the 15th day of pregnancy, the rats were divided into 3 groups: 1. 1st group (intact) - 30 pregnant rats, 2. 2nd group (comparisons) - 30 pregnant rats, 3. 3rd group (main) - 30 pregnant rats.From the 16th to the 19th day of gestation, daily sesame oil was administered subcutaneously to females of the 2nd group at a dose of 1.0 ml, whereas females of the 3rd group were injected subcutaneously with 5 mg of “Testosterone” to females of the 3rd group (T-1500; Sigma) dissolved in 1.0 ml of sesame oil. Each pregnant female rat was set apart separately. Births occurred on the 20-22nd days of pregnancy. The rats were selected from the offspring in such a way as to equalize the size of groups (10 calves (T2) per mother (T1)).On the 30th day after the birth, the female cubs were separated from the male calves. At the same time, T2 females in the 2nd and 3rd groups were divided into 2 subgroups of 48 in each:1. subgroup A (T2) (n = 32) received a high-calorie diet (HCD) (5.24 kcal / g: 60% fat, 20% carbohydrate, 20% protein) and water on demand;2. subgroup B (T2) (16 female rats each) - normal (RD) (3.30 kcal / g: fat 15%, carbohydrates 62%, proteins 23%) diet [7. 9].During the experiment, animals were slaughtered by decapitation under light ether anesthesia on the 60th day from birth (in 1-group-10 (T2) females, in the 2nd A and B subgroups of 6 (T2) females, in the 3rd A - 7 (T2) females in the 3rd B subgroup - 6 (T2) females.To achieve this goal, this study carried out studies of hormones in the blood serum of animals by enzyme immunoassay (ELISA) on an enzyme immunoassay analyzer from Human (Germany) using standard kits of the same company. The content of prolactin (PRL), luteinizing hormone (LH), follicle-stimulating hormone (FSH), estradiol (Est), total testosterone (TTS), cortisol (Cort) and dehydroepiandosterone sulfate (DHEA-s) was determined.
2.3. Results and Discussion
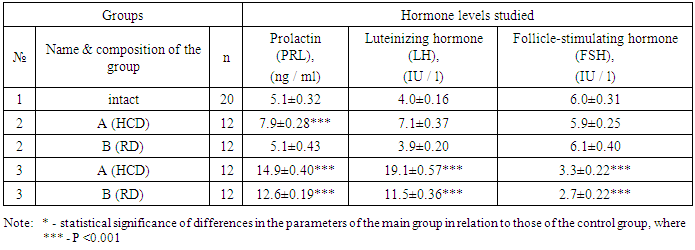
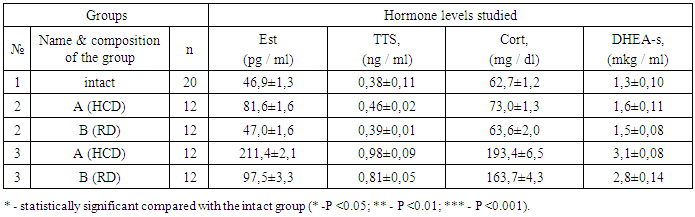
- The results of a comparative analysis of the level of the main hormonal parameters showed that in prenatally hyperandrogenized rats the mean serum level of PRL increased significantly (P <0.001), while there was some difference in the values between subgroups A (14.9 ± 0.40 ng / ml) and B (12.6 ± 0.19 ng / ml). The level of PRL in the 3rd group exceeded that in the 1st and in the 2nd group on average 2.7 and 2.1 times, respectively. A high level of PRL in prenatally hyperandrogenized animals is explained by a violation of the regulation of its synthesis and secretion, which is especially pronounced in rats that were on a lipid diet.The average levels of LH in animals of the 2A subgroup were significantly higher, the average values in the subgroup 2B (3.9 ± 0.2 IU / l; P <0.001) in the intact group (4.0 ± 0.16 IU / l) and on average amounted to 7.1 ± 0.37 IU / l (P <0.001). In 3A (19.1 ± 0.57 IU / l; P <0.001) and 3B subgroups (11.5 ± 0.36 IU / l; P <0.001) the level of LH significantly exceeded the corresponding figure in the 1st group on average 3.8, and in the 2nd combined group on average 2.8 times. The increase in LH in the group of prenatally androgenated rats is probably associated with the stimulation of its production by the pituitary gland, as indicated by the literature data [12]. Along with the above changes, the average FSH level of 3 A (3.3 ± 0.22 IU / l; P <0.001) and 3 B (2.7 ± 0.22 IU / l; P <0.001) of the subgroups tended to decrease in relation to animals of the intact and 2nd group (Table 1.). These differences, characterized by a decrease in FSH in prenatally hyperandrogenic animals, indicate a violation of the folliculogenesis period, in which the basal growth of follicles occurs only in the presence of a sufficient level of FSH. In our study, disorders of folliculogenesis are confirmed by morphological studies of the structure of the ovaries.Conducted correlation analysis established a direct correlation of LH with PRL (r = 0.14), FSH (r = 0.36), TTS (r = 0.27) and DHEA-s (r = 0.16), FSH with TTS and TTS (r = 0.25) and DHEA-s (r = 0.27) (Table 1.).
|
|
3. Conclusions
- 1. Prenatal hyperandrogenization in rats in the postnatal period of development leads to an imbalance of the endocrine profile.2. The most pronounced changes among the indicators of the endocrine profile are observed in rats that were on a high-calorie diet (HCD), while HCD is an additional factor aggravating endocrinological disorders.
ACKNOWLEDGEMENTS
- The author thanks the scientific adviser and the head of the Department of Molecular Medicine and Cell Technologies of, Scientific Research Institute of Hematology and Blood Transfusion of the Ministry of Health of the Republic of Uzbekistan Khamid Yakubovich Karimov for assistance in the management and organization of the conducted studies.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML