-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2019; 9(1): 20-27
doi:10.5923/j.ajmms.20190901.04

The Effect of Human Embryonic Hepatocytes on Biochemical Indices at Acute Hepatic Failure in the Experiment
A. G. Mirzakulov, M. D. Urazmetova, F. A. Khadjibaev
Republican Research Center of Emergency Medicine, Tashkent, Uzbekistan
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Acute hepatic failure has been simulated in the rats by single intraperitoneal introduction of CCl4 hepatotrophic toxin in a dose of 1 ml/kg. Human embryonic hepatocytes were extracted from human embryo (spontaneous abortions). Fifty million (50×106) fresh preparated hepatocytes were transplanted intraabdominally in the period between 30 and 34 hours after induction of acute hepatic failure. The following biochemical indices of liver functional condition were defined: glucose, crude protein, albumine, urea, creatinine, general bilirubin, AST, ALT, alkaline phosphatase, ammonia. At acute hepatic failure the decrease of some indices (glucose, the crude protein, albumine) and simultaneous increase of the others (urea, creatinine, the general bilirubin, AST, ALT, alkaline phosphatase, ammonia) reflecting changes in the liver functioning has been noted. Human embryonic hepatocytes corrects liver dysfunctions in the rats with acute hepatic failure starting from 7 days after treatment.
Keywords: Human embryonic hepatocytes, Acute hepatic failure, Transplantation of hepatocytes, Biochemical indices
Cite this paper: A. G. Mirzakulov, M. D. Urazmetova, F. A. Khadjibaev, The Effect of Human Embryonic Hepatocytes on Biochemical Indices at Acute Hepatic Failure in the Experiment, American Journal of Medicine and Medical Sciences, Vol. 9 No. 1, 2019, pp. 20-27. doi: 10.5923/j.ajmms.20190901.04.
Article Outline
1. Introduction
- Acute hepatic failure (AHF) is a relevant issue of practical medicine and hepatology [1, 6]. There are many etiological factors leading to its development such as: fulminant forms of viral hepatitis, drug intoxication, severe intoxications by poisons and alcohol, sepsis, massive burns, shock of different etiology, acute suprarenal failure, cardio-surgical interventions, surgical diseases of hepatopancreatobiliary system, metabolic disorders and etc. [2, 9].The majority of clinicians consider the basis of AHF pathogenesis is an evident humoral and hyperimmune response caused by massive necrosis of hepatocytes. Its severity is directly depends on hepatocytes percentage which lost their functional and reserve abilities and also depends on the duration of the period of their death [5, 8].Disabled hepatocytes due to the change of membrane proteins structure are apprehended as foreign ones by the immune system and are destroyed. But it is necessary to mention that the liver has big compensatory abilities. As a rule, the AHF development points that more than 75-80% of hepatocytes are disabled [9].The treatment of AHF is very complicated problem. The most effective treatment method is a liver transplantation, but it has some disadvantages (the lack of donor organs, the necessity of immunosuppressants constant taking, a high risk of severe postoperative complications development) [7].With the aim of prevention and treatment of AHF the meal of embryonic hepatocytes providing detoxicating effect, activating regenerative processes and improving the prognosis of the disease has been begun to use for the recent years [3, 10]. Earlier we studied a condition of the immune system of animals from ALF receiving HEH [4].
2. Aim
- To estimate the dynamics of biochemical indices at the treatment of acute hepatic failure in the rats with the use of human embryonic hepatocytes.
3. Material and Methods
- The AHF model was reproduced in the laboratory rats (males) by a single intraperitoneal introduction of CCl4 hepatotrophic toxin in a dose of 1 ml/kg. In the experiment we used mature outbred male rats. The main criteria for the inclusion of animals in the study were: body weight 230-270 g; age 23-24 weeks; wool - smooth, shiny; behavior and general condition - the active dynamics of movement and feed consumption. Before the start of the study, rats meeting these criteria were divided into groups using the randomization method. The study protocol was approved by the National Ethics Committee (No. 4 of April 13, 2011), and in the course of the experiment, the requirements of the World Society for the Protection of Animals (WSPA) and the European Convention for the Protection of Vertebrate Animals used for experimental and other scientific purposes (Strasbourg, 1986).Human embryonic hepatocytes were obtained from abortive material with the obligatory consent of the mother, while conducting tests for a wide range of viral diseases.As a source of donor cells the liver of embryos of non-viable human fetuses, weighing less than 500 grams was obtained from women who artificially terminate the pregnancy for medical reasons at gestational age of 8-11 and 16-20 weeks. The embryo was sterilely transferred to a 250 ml glass bottle with Hanks solution containing the antibiotic gentamicin at a concentration of 500 mcg / ml. Then the embryo was washed three times by Hanks solution with kanamycin (50 mcg / ml).Human embryonic hepatocytes were extracted from human embryo (spontaneous abortions).The method of obtaining embryonic hepatocytes was based on the enzymatic-mechanical method of processing the original tissue with minimization of manipulations damaging the embryonic hepatocytes (EH). The essence of the method is to increase the percentage of yield of viable EH with increased morphofunctional properties and reduce the time to receive them.The method of obtaining the EH included: laparotomy, portal vein cannulation, liver perfusion with nutrient solutions in 2 stages, then the liver was removed from the abdominal cavity, the liver was mechanically processed by chopping the liver with scissors into pieces up to 0.7 mm thick which were transferred into a glass with phosphate-buffered saline 0.25% solution of proteolytic enzyme trypsin, placed on a magnetic stirrer for 15 minutes at + 37°C. After enzymatic treatment, medium 199 is added to inactivate trypsin. Next was performed homogenization and filtration.Experimental animals were kept in accordance with the established standards of the World Society for the Protection of Animals (WSPA) and the European Convention for the Protection of Vertebrate Animals used for experimental and other scientific purposes. Animals were kept by 4-5 heads in a cage with a sufficient amount of lighting, power, access to water. Statistical processing of research results was carried out on the basis of methods of variation statistics using parametric Student's t-test and non-parametric Mann-Whitney U-test.For investigation of the general bilirubin, albumine and ammonia a blood sampling was taken from the tail of the rats. Thecontent of some biochemical indices in the blood serum was determined by the unified methods accepted in clinical practice (V.G. Kolb, V.S. Kamyshnikov, 1982). The content of urea was determined by staining reaction with diacetylmonooc sim, creatinine - by staining reaction of Jaffe (the method of Popper and al.), the general bilirubin –by a colorimetric method of Yendrashik, Kleggorn, Groff. In the animals blood serum we determined the following indices: concentration of the crude protein (CP), general bilirubin, urea, glucose, activity of aspartate aminotransferase (AST), alaninaminotranspherase (ALT), alkaline phosphatase (AP). Biochemical researches were carried out with the use of “Vitros” analyzer (Japan).Animals were divided into groups by 10 in each. The first group was consisted of the rats with the AHF model in which biochemical researches were conducted 3, 7 and 14 days after simulating AHF. The second comparative group was made by the animals with AHF treated by means of HEH without additional introduction of immunosuppressors. Researches were conducted 7 and 14 days after simulating AHF.
4. Results and Discussion
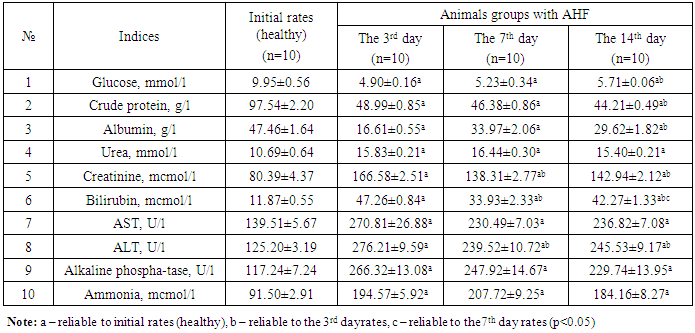
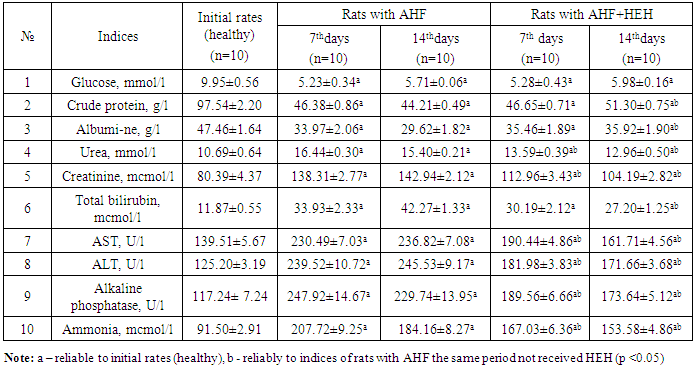
- Dynamic biochemical researches in the rats with AHF showed the following results (tab. 1).
|
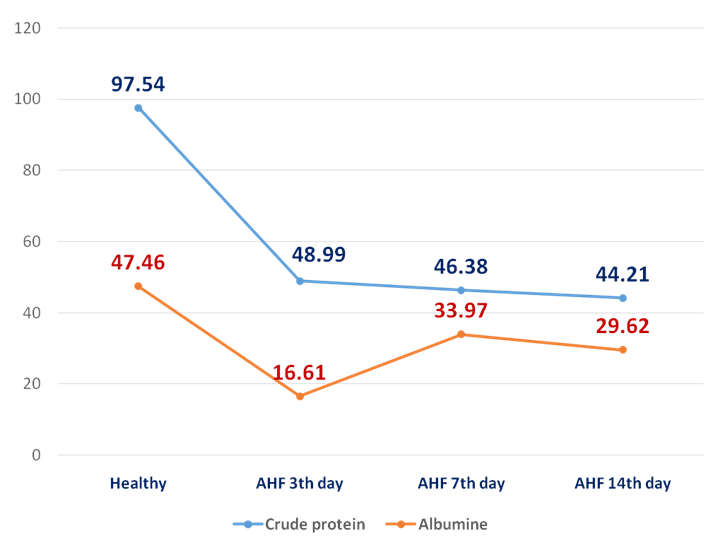 | Figure 1. The dynamics of crude protein and albumine (g/l) in the rats with AHF on the 3rd, 7th, 14th day |
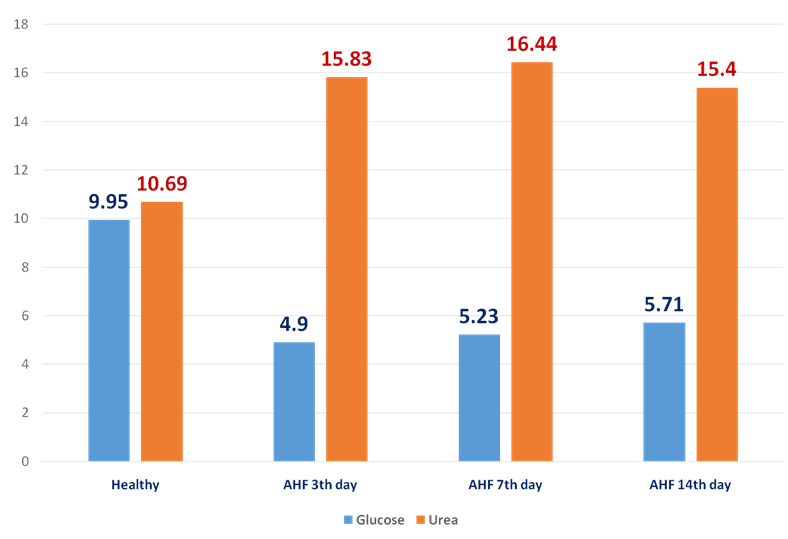 | Figure 2. The dynamics of glucose and urea (mmol/l) in the rats with AHF on the 3rd, 7th, 14th day |
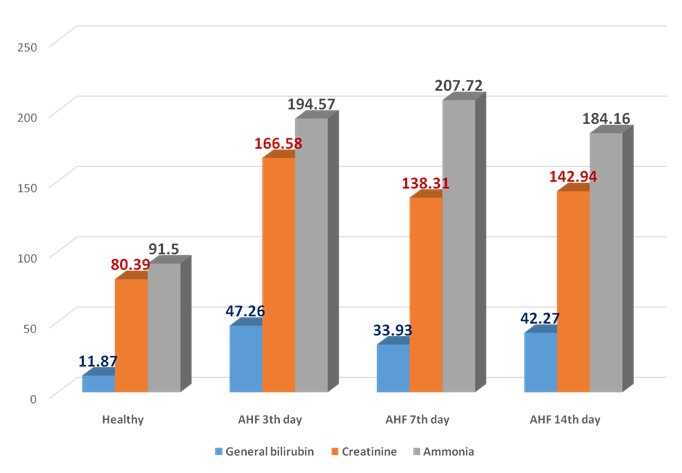 | Figure 3. The dynamics of general bilirubin, creatinine, ammonia (mcmol/l) in the rats with AHF on the 3rd, 7th, 14th day |
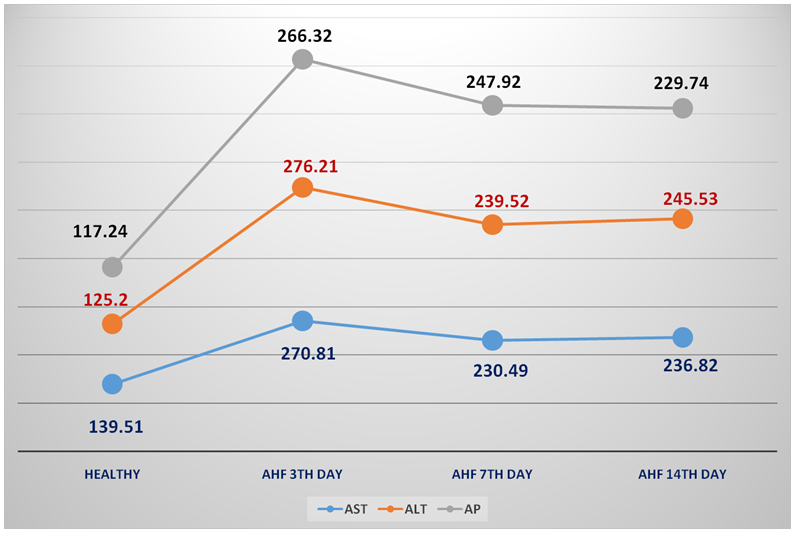 | Figure 4. The dynamics of AST, ALT and AP (U/l) in the rats with AHF on the 3rd, 7th, 14th day of the experiment |
|
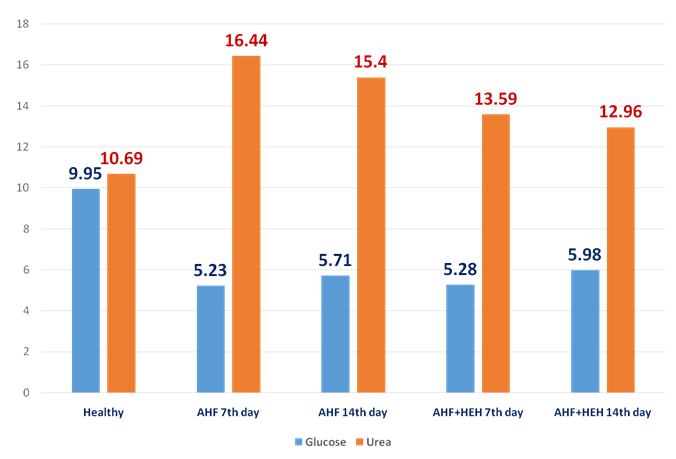 | Figure 5. The dynamics of glucose and urea (mmol/l) in the rats with AHF modeling and treated by HEH on the 3rd, 7th, 14th day of the experiment |
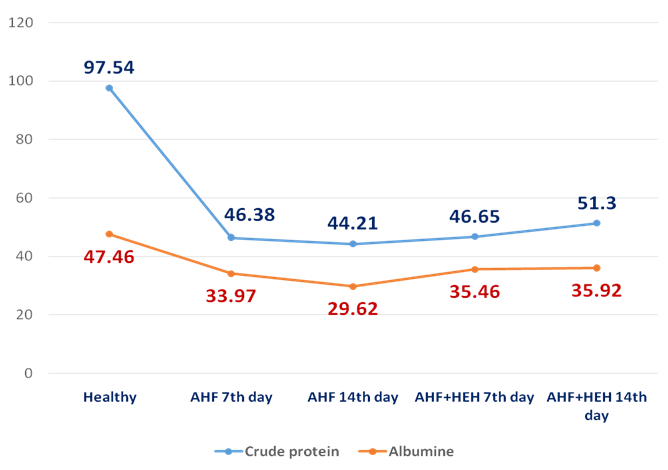 | Figure 6. The dynamic of crude protein and albumine (g/L)I n the rats with AHF modeling and treated by HEH on the 3rd, 7th, 14th day of the experiment |
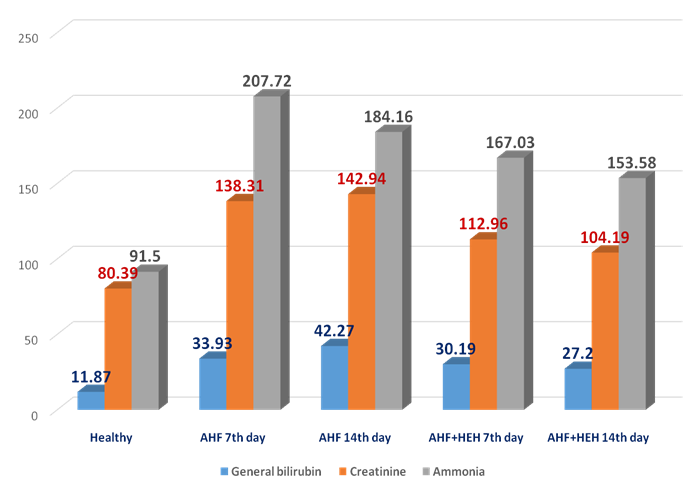 | Figure 7. The dynamics of general bilirubin, creatinine and ammonia (mcmol/l) in the rats with AHF modeling on the 3rd, 7th, 14th day of the experiment |
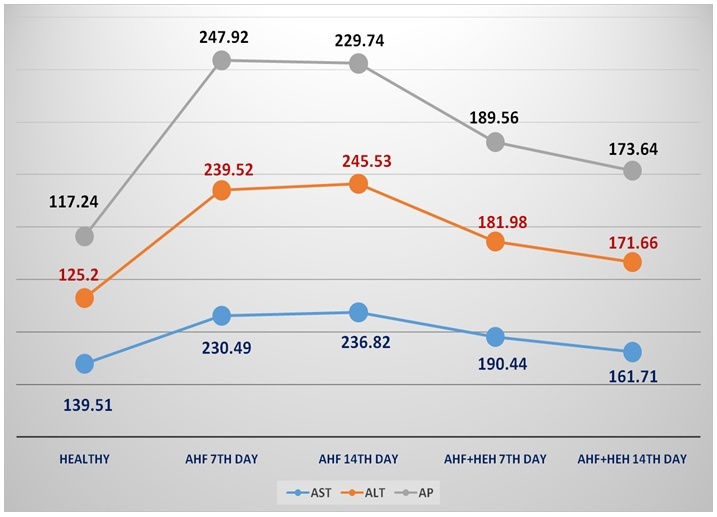 | Figure 8. The dynamics of AST, ALT and AP (u/L) in the rats with AHF modeling and treated by HEH on the 3rd, 7th, 14th day of the experiment |
5. Conclusions
- In the animals with AHF the decrease of some biochemical indices (glucose, the crude protein, albumine) and simultaneous increase of the others (urea, creatinine, the general bilirubin, AST, ALT, alkaline phosphatase, ammonia) which reflect changes in liver functioning was noted. Under the influence of HEH the normalization of biochemical characteristics in the rats with AHF occurs starting with the 7th day after treatment.
Abbreviations
- AHF - acute hepatic failureHEH - human embryonic hepatocytesAST - aspartate aminotransferase ALT - alaninaminotranspheraseAP - alkaline phosphatase
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML