-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2019; 9(1): 7-13
doi:10.5923/j.ajmms.20190901.02

Chemerin and Vaspin as Noninvasive Biomarkers in the Pathogenesis and Diagnosis of Non- Alcoholic Fatty Liver Disease
Sahar Mohamed Ismail 1, Naglaa Atef Elgendy 2, Zeinab H. El Sayed 1, Aida Ahmed Abd El Hameed 3, Inass Hassan Ahmad 4, Azza Ali Althoqapy 5
1Department of Internal Medicine, Faculty of Medicine – Girls, Al-Azhar University, Cairo, Egypt
2Department of Tropical Medicine, Faculty of Medicine – Girls, Al- Azhar University, Cairo, Egypt
3Department of Clinical Pathology, Faculty of Medicine – Girls, Al –Azhar University, Cairo, Egypt
4Department of Endocrinology, Faculty of Medicine – Girls, Al- Azhar University, Cairo, Egypt
5Department of Microbiology and Immunology, Faculty of Medicine – Girls, Al –Azhar University, Cairo, Egypt
Correspondence to: Zeinab H. El Sayed , Department of Internal Medicine, Faculty of Medicine – Girls, Al-Azhar University, Cairo, Egypt.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background: Many challenges facing the accurate diagnosis of progression of Nonalcoholic fatty liver disease (NAFLD). Liver biopsy is the only accurate test. Chemerin and vaspin are adipokines that may play a role in the pathogenesis of simple steatosis and Nonalcoholic steatohepatitis (NASH). We aimed to evaluate the role of chemerin and vaspin in pathogenesis and diagnosis of simple steatosis and NASH as non-invasive biomarkers in obese patients. Methods: Thirty obese NAFLD patients included in our study were divided into two groups: simple steatosis (n=fifteen patients), and NASH (n=fifteen patients). Another fifteen nonobese subjects who were apparently healthy served as a control group (CG). We determined serum of chemerin, vaspin, insulin, alanine transaminase (ALT), aspartate aminotransferase (AST), fasting blood glucose and HOMA-IR. Results: Chemerin was significantly elevated in the simple steatosis and NASH groups compared to CG, but there were no significant differences between simple steatosis and NASH. Positive associations with chemerin were observed for BMI, fasting insulin levels, triglycerides, total cholesterol, HOMA-IR, ALT, and AST. Vaspin was significantly decreased in simple steatosis and NASH groups, compared to the CG, while there were no significant differences between simple steatosis and NASH. There was no correlation of vaspin with all the parameters in the simple steatosis and NASH groups. Conclusion: We confirmed that the chemerin, and not the vespin levels, is strongly associated with obese NAFLD. Chemerin and vespin may play a role in the pathogenesis of NAFLD, but they do not play a role in the progression of simple steatosis.
Keywords: Biomarkers, Chemerin, Non-alcoholic fatty liver disease, Pathogenesis, Vaspin
Cite this paper: Sahar Mohamed Ismail , Naglaa Atef Elgendy , Zeinab H. El Sayed , Aida Ahmed Abd El Hameed , Inass Hassan Ahmad , Azza Ali Althoqapy , Chemerin and Vaspin as Noninvasive Biomarkers in the Pathogenesis and Diagnosis of Non- Alcoholic Fatty Liver Disease, American Journal of Medicine and Medical Sciences, Vol. 9 No. 1, 2019, pp. 7-13. doi: 10.5923/j.ajmms.20190901.02.
Article Outline
1. Introduction
- Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease all over the world [1] and is commonly associated with obesity, dyslipidemia and insulin resistance [2]. It usually has no symptoms, in most cases, and it diagnosed accidentally when investigations were performed for other reasons [3, 4]. On the bases of disease severity, NAFLD is divided into simple steatosis and a more severe form called nonalcoholic steatohepatitis (NASH), which progresses to liver fibrosis and cirrhosis faster than simple steatosis [5].The patients with NASH are at a high risk of developing cardiovascular disease, chronic kidney disease, and type 2 diabetes mellitus, besides liver complications [6]. Although many studies recognize that NASH is an important cause of decompensated liver disease and hepatocellular carcinoma, the diagnosis by noninvasive techniques gives a suboptimal performance. The liver biopsy is still the only accurate way for the diagnosis and differentiation between simple steatosis and NASH, but it is not possible to routinely carry out biopsies, and biopsies are associated with risks [7]. Therefore, we need new, accurate, and noninvasive biomarkers to help in the early diagnosis of NAFLD and NASH.Chemerin and vaspin are adipokines produced by the liver cells and adipose tissue [8]. They have a strong relation with obesity, insulin resistance, and atherosclerosis. Recently, there is growing evidence that chemerin and vaspin may play a role in the progression from simple steatosis to inflammation and fibrosis [9]. However, an accurate assessment of the role of chemerin and vaspin in the diagnosis is still lacking. Therefore, the aim of our study is to evaluate the role of chemerin and vaspin in the pathogenesis and diagnosis of simple steatosis and NASH as non-invasive markers in obese patients.
2. Patients and Methods
- Our study included thirty obese NAFLD patients who visited the tropical and internal medicine outpatient’s clinics between November 2017 and March 2018. The patients were diagnosed and divided into two groups; a simple steatosis group and a NASH group, based on the evidence of fatty changes on abdominal ultrasonography [10], and on the HAIR score [11] (hypertension, elevation of alanine transaminase (ALT), insulin resistance), to the exclusion of liver diseases of other etiologies. There were another fifteen apparently healthy non-obese participants serving as a control group. Informed written consent was obtained from all participants and was conducted according to the declaration of Helsinki. Our study was approved by the local Ethics Committee at Al-Azhar University.Ÿ Control group: their age ranged from 35 to 70 years with a mean ± SD (50.20±9.10) years. BMI ranged between≥ 20<25 kg/m2. All were females.Ÿ Simple steatosis group: this includes fifteen obese (BMI>30 kg/m2) patients who had normal serum AST and ALT levels. Their age ranged between 40 to 74 years with a mean ±SD (47.93±4.31) years. All of them were females.Ÿ NASH group: this included fifteen obese (BMI≥30 kg/m2) NASH patients who had a persistent elevation of serum ALT and AST. Their age ranged from 35 to 70 years with a mean ±SD (50.20±9.10). Ten of them were females.Exclusion criteria:We exclude all patients who had acute or chronic hepatitis, chronic renal disease, patients who had received metformin, had a malignancy, active infection or consumed alcohol.Methods:Ÿ A full comprehensive history and a full clinical examination were taken for all participants.Ÿ Anthropometric measurements:Individual weight was measured/kg and height was measured/cm. Body mass index (BMI) was calculated by
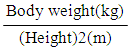 Ÿ Laboratory tests:A complete blood count, alanine transaminase (ALT) Iu/l, aspartate aminotransferase (AST)Iu/l, gamma-glutamyl transferase, serum albumin g/dl, anti-hepatitis C virus antibodies, and hepatitis B surface antigen. Fasting blood sugar mg/dl, serum triglycerides, total cholesterol, blood urea, and serum creatinine were done at Al Zahraa hospital. Serum vaspin ng/ml, serum chemerin ng/ml, and fasting serum insulin level uIu/ml were performed at Al-Azhar University Center for Virus Research and Studies.Ÿ Insulin resistance, it was estimated by the homeostasis model assessment for insulin resistance (HOMA-IR)
Ÿ Laboratory tests:A complete blood count, alanine transaminase (ALT) Iu/l, aspartate aminotransferase (AST)Iu/l, gamma-glutamyl transferase, serum albumin g/dl, anti-hepatitis C virus antibodies, and hepatitis B surface antigen. Fasting blood sugar mg/dl, serum triglycerides, total cholesterol, blood urea, and serum creatinine were done at Al Zahraa hospital. Serum vaspin ng/ml, serum chemerin ng/ml, and fasting serum insulin level uIu/ml were performed at Al-Azhar University Center for Virus Research and Studies.Ÿ Insulin resistance, it was estimated by the homeostasis model assessment for insulin resistance (HOMA-IR) 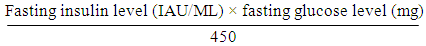 Ÿ Abdominal ultrasound and its evaluation were performed by an experienced radiologist using a Philips- Affinti 70 G.Sample collectionFrom each subject and control, fasting peripheral venous blood samples were collected under complete aseptic conditions. Seven ml of venous blood were withdrawn and divided as follows:A- 2ml anticoagulated with EDTA was used immediately for a complete blood count. This was performed by the fully automated cell counter Sysmex KX-2IN.B- 5ml was put in a plan tube, left to clot then centrifuged at 1000xg for 5 minutes and serum was separated. Part of the sera were used immediately for routine laboratory tests; anti-hepatitis C virus antibodies HCVAb, hepatitis B surface antigen HBSAg and HIV antibody, cholesterol, triglyceride, kidney function test, liver enzymes (ALT, AST) fasting blood sugar, total cholesterol (TC), triglyceride (TG), kidney function test (all chemistry investigation were done on Cobase c311 auto –analyzer using (Roche-reagent kits.Hitachi, Japan)). The remaining serum sample was removed in aliquots and stored at -70 °C until assays of adepokines (chemerin & vaspin) and serum insulin stored at -20 °C. Adipokines and Insulin measurement:Serum levels of adipokines (chemerin, andvaspin) and serum insulin were measured by enzyme-linked immune-sorbent assay (ELISA) uses Sandwich –ELISA as the method using commercial kits: Human visceral adipose-specific serine protease inhibitor (vaspin) ELISA kit (Cat No. In- Hu3180: China Bioneovan Co., Ltd.), and Human chemerin ELISA kit (Cat No. In- Hu1852: China Bioneovan Co., Ltd.) Human insulin ELISA kit (Cat No.10801: USA Perfect Ease Biotech (Beijing) Co., Ltd.) (16. Briefly, standards, samples and controls were pipetted into wells, precoated with specific Antibody, and any vaspin or chemerin and insulin present was bound to the immobilized antibody. After removing any unbound substances. After washing, Horseradish Peroxidase (HRP) conjugated specific antibody for vaspin or chemerin or insulin was added to the wells. Following wash to remove any unbound conjugate-enzyme reagent, a substrate solution (TMB) was added to the wells and color developed in proportion to the amount of vaspin or chemerin or insulin bound in the initial step. The color development was stopped and the intensity of the color was measured (Read at 450nm wave length using Stat-Fax 2100, AWARENESS TECHNOLOGY INC.ELISA reader). A standard curve was prepared relating color intensity to the concentration. The levels were calculated from the standard curve corresponding to the measured optical density. The results were expressed as ng/ml for vaspin, ng/ml, pg/ml for chemerin, µiU/ml for insulin.The intra- and inter-assay coefficient of variation (CV) of vaspin and chemerin was ranged from < 10% to < 12%, respectively. The assay range for vaspin is (0.3 - 20 ng/ml). The assay range for chemerin is (20pg/mL-1200 pg /ml). The sensitivity of insulin was 2 µIU/mL.Expected value for insulin: adult (normal) (0.7-9.0 µIU/ml).Diabetic (type2) (0.7-25 µIU/ml).
Ÿ Abdominal ultrasound and its evaluation were performed by an experienced radiologist using a Philips- Affinti 70 G.Sample collectionFrom each subject and control, fasting peripheral venous blood samples were collected under complete aseptic conditions. Seven ml of venous blood were withdrawn and divided as follows:A- 2ml anticoagulated with EDTA was used immediately for a complete blood count. This was performed by the fully automated cell counter Sysmex KX-2IN.B- 5ml was put in a plan tube, left to clot then centrifuged at 1000xg for 5 minutes and serum was separated. Part of the sera were used immediately for routine laboratory tests; anti-hepatitis C virus antibodies HCVAb, hepatitis B surface antigen HBSAg and HIV antibody, cholesterol, triglyceride, kidney function test, liver enzymes (ALT, AST) fasting blood sugar, total cholesterol (TC), triglyceride (TG), kidney function test (all chemistry investigation were done on Cobase c311 auto –analyzer using (Roche-reagent kits.Hitachi, Japan)). The remaining serum sample was removed in aliquots and stored at -70 °C until assays of adepokines (chemerin & vaspin) and serum insulin stored at -20 °C. Adipokines and Insulin measurement:Serum levels of adipokines (chemerin, andvaspin) and serum insulin were measured by enzyme-linked immune-sorbent assay (ELISA) uses Sandwich –ELISA as the method using commercial kits: Human visceral adipose-specific serine protease inhibitor (vaspin) ELISA kit (Cat No. In- Hu3180: China Bioneovan Co., Ltd.), and Human chemerin ELISA kit (Cat No. In- Hu1852: China Bioneovan Co., Ltd.) Human insulin ELISA kit (Cat No.10801: USA Perfect Ease Biotech (Beijing) Co., Ltd.) (16. Briefly, standards, samples and controls were pipetted into wells, precoated with specific Antibody, and any vaspin or chemerin and insulin present was bound to the immobilized antibody. After removing any unbound substances. After washing, Horseradish Peroxidase (HRP) conjugated specific antibody for vaspin or chemerin or insulin was added to the wells. Following wash to remove any unbound conjugate-enzyme reagent, a substrate solution (TMB) was added to the wells and color developed in proportion to the amount of vaspin or chemerin or insulin bound in the initial step. The color development was stopped and the intensity of the color was measured (Read at 450nm wave length using Stat-Fax 2100, AWARENESS TECHNOLOGY INC.ELISA reader). A standard curve was prepared relating color intensity to the concentration. The levels were calculated from the standard curve corresponding to the measured optical density. The results were expressed as ng/ml for vaspin, ng/ml, pg/ml for chemerin, µiU/ml for insulin.The intra- and inter-assay coefficient of variation (CV) of vaspin and chemerin was ranged from < 10% to < 12%, respectively. The assay range for vaspin is (0.3 - 20 ng/ml). The assay range for chemerin is (20pg/mL-1200 pg /ml). The sensitivity of insulin was 2 µIU/mL.Expected value for insulin: adult (normal) (0.7-9.0 µIU/ml).Diabetic (type2) (0.7-25 µIU/ml).2.1. Statistical Methods
- Collected and recorded data were analyzed using the statistical package for social science (IBM SPSS), version 23. Quantitative data were expressed as a mean± standard deviation. A test to compare quantitative data of more than two groups was performed by the analysis of variance test.
3. Results
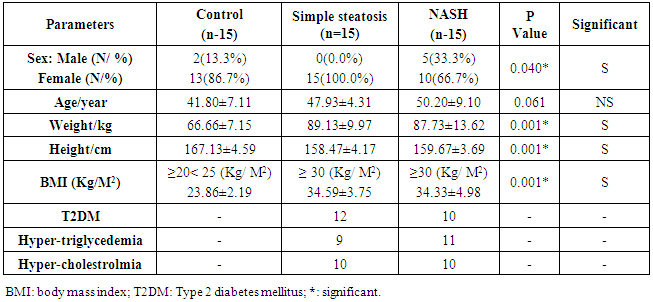
- Table 1 shows the comparative study between all groups by using ANOA test according the clinical and anthropometric data. The simple steatosis group (n=15 patients), 12 of them had T2DM, 9 had hypertriglyceridemia, and 10 had hypercholesterolemia. The NASH group (n=15 patients), 10 had T2DM, 11 had hypertriglyceridemia, and 10 had hypercholesterolemia. There were significant differences in age, weight, height, and BMI between the groups.
|
|
|
|
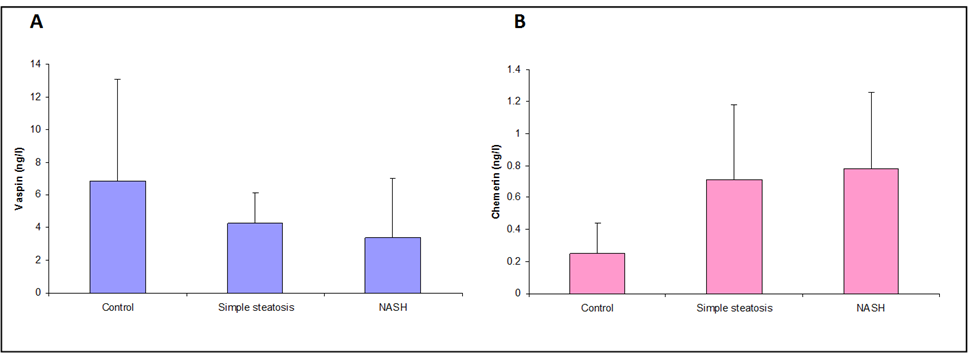 | Figure 1. A. Vaspin statuses in group’s studies. B. Chemerin statuses in group’s studies |
4. Discussion
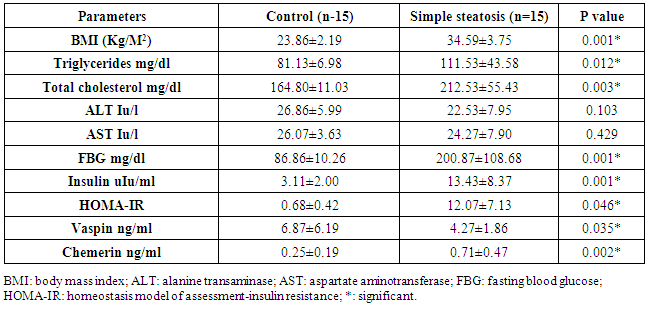
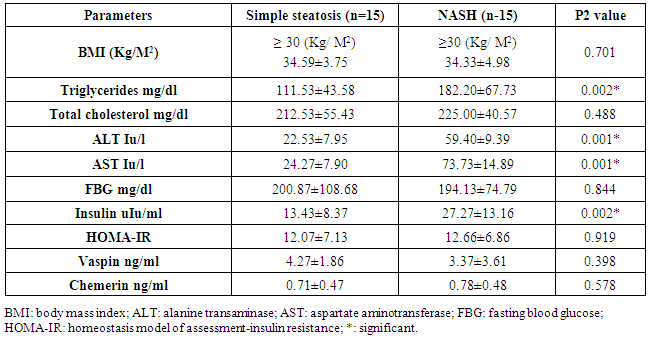
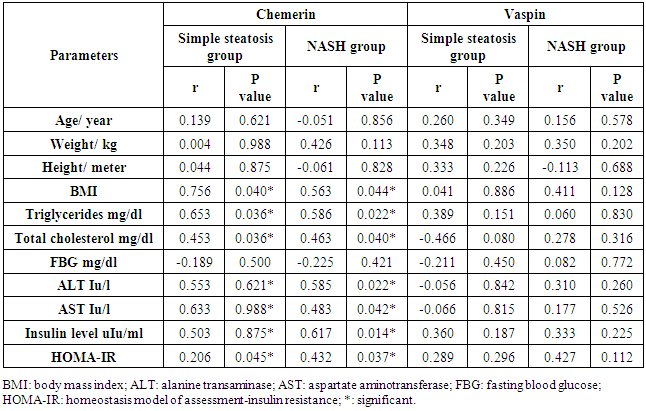
- The data regarding the role of chemerin and vaspin in NAFLD is limited, particularly in obese patients. The adipokines are in balance when the adipose tissue is in the normal range, but the balance tends to be disrupted when the adipose tissues expand. Our study aimed to evaluate the role of chemerin and vaspin in the pathogenesis and diagnosis of simple steatosis and NASH as non-invasive markers in obese patients.Our results demonstrated that the circulating chemerin was significantly elevated and vaspin was significantly reduced in the obese simple steatosis and NASH groups compared to non-obese healthy control subjects. However, both chemerin and vaspin for simple steatosis and NASH patients were not significantly different. In addition, chemerin was positively correlated with body mass index, triglyceride, total cholesterol, ALT, AST, insulin level, and HOMA-IR in the obese simple steatosis and NASH groups. However, we did not find any correlation of vaspin with body mass index, triglyceride, total cholesterol, ALT, AST, insulin level, and HOMA-IR in the obese simple steatosis and NASH groups.These findings indicate that obese patients with elevated chemerin and reduced vaspin are at an increased risk of developing NAFLD. Therefore, the chemerin and vaspin play important roles in the pathogenesis of NAFLD. This result is in accordance with other studies that found that NAFLD was associated with high circulating chemerin and low circulating vaspin levels [12-14]. Furthermore, a recent study by Kukla et al. [15] showed an increase in the chemerin level in morbidly obese NAFLD women, and the chemerin level in hepatic vein samples was higher than that in systemic circulation in cirrhotic patients, which indicated that the chemerin was produced by the liver as by the adipose tissue. Another study reported that chemerin was appositively correlated with total cholesterol, triglycerides, fasting insulin and the high sensitive C-reactive protein [16, 17]. Therefore, chemerin appears to be related to some features of the metabolic syndrome, and plays a role in the pathogenesis of NAFLD [18]. This could be explained by obesity being a state of low-grade subclinical systemic inflammation, with the presence high circulating prion, inflammatory cytokines; this inflamed adipose tissue might play a role in increased chemerin secretion by adipocytes [19]. Moreover, chemerin induces the initiation of the innate immune response and inflammatory processes by attracting macrophages. Therefore, the pro-inflammatory and immune-modulating properties of chemerin may be closely associated with simple steatosis pathogenesis [18]. The elevation of chemerin with NAFLD may be explained by the adipocytes being responsible for storing excess calories. Therefore, any energy imbalance leads to adipocyte hyperplasia, which leads to increased circulatory prion-inflammatory cytokines [19]. Furthermore, elevated serum chemerin may increase the insulin resistance through the reducing effect of chemerin on the insulin receptor signaling cascades, which leads to insulin resistance or aggravates it; this was confirmed by the association between chemerin and HOMA-IR [20]. On the other hand, few authors reported no significant difference in chemerin levels between NAFLD and the control groups [21]. However, Buechler et al [22] reported that the chemerin was reduced in hepatic cirrhosis. Moreover, another study found no significant correlations between serum chemerin levels with fasting blood glucose and HOMA-IR [23]. Our study indicated that chemerin plays a role in the progression of simple steatosis to NASH and another study suggested that the chemerin plays a role in hepatocyte ballooning degeneration in NAFLD patients, which indicates a possible connection between liver chemerin and the development of NASH [24]. However, in our study, we did not find that chemerin plays any role in the progression of simple steatosis to NASH. In the case of vaspin, a study by Polygons et al [25] found low values of circulating vaspin in NAFLD patients compared to the control, but the amount of circulating vaspin was similar in simple steatosis and NASH patients, which was in line with our result. While Kukla M. et al [26] found the amount of vaspin was less in simple steatosis patients compared to the control, but there was a significant increase in vaspin in obese (BMI ≥40) NASH and in advanced liver fibrosis patients compared to simple steatosis. Also, they [26] did not find any association between vaspin and lipid parameters in NAFLD. Unfortunately, our study did not include liver fibrosis or cirrhosis. However, Genc et al. [27] showed there was no significant difference in the serum concentration of vaspin between patients with NAFLD and healthy controls. On the other hand, some authors found higher vaspin levels in NAFLD patients compared to healthy controls [28]. Therefore, our result with regard to vaspin revealed that a low level of vaspin was a risk factor for NAFLD development, which could be explained by the anti-inflammatory and anti-oxidant effect of vaspin [29]. The limitation of our study: 1. The number of patients in the study sample was small.2. A limitation arose from the NAFLD patients who disagreed to perform a liver biopsy.
5. Conclusions
- Our study confirmed that the chemerin level, and not the vespin level, is strongly associated with obese NAFLD patients and metabolic syndrome elements. Both vespin and chemerin may play a role in the pathogenesis of NAFLD, but they did not play a role in the progression from simple steatosis to NASH. Therefore, we cannot depend on either of the previous biomarkers to differentiated NASH from simple steatosis, and a liver biopsy remains the more accurate method for diagnosis of NASH. Further studies will be required: (I) to understand why some simple steatosis patients progress to NASH, and another to learn why some simple steatosis patients can progress. (II) Additional study is needed with a large number of patients which also contain lean non-diabetic NAFLD patients.
ACKNOWLEDGEMENTS
- The authors would like to thank the staff of the radiological department, who was helpful in performing and interpreting the abdominal ultrasound.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML