-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2019; 9(1): 1-6
doi:10.5923/j.ajmms.20190901.01

Study of the Effectiveness of the Use of the Drug Immunoglobulin for Intravenous Administration of "Octagam" as Part of a Complex Treatment of Hematological Diseases in Uzbekistan
Feruza Abdulkhamitovna Rizaeva, Khamid Yakubovich Karimov
Department of Pediatric Hematology, Scientific Research Institute of Hematology and Blood Transfusion of the Ministry of Health of the Republic of Uzbekistan, Tashkent, Uzbekistan
Correspondence to: Feruza Abdulkhamitovna Rizaeva, Department of Pediatric Hematology, Scientific Research Institute of Hematology and Blood Transfusion of the Ministry of Health of the Republic of Uzbekistan, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The aim of the study was to assess the tolerability, safety and efficacy of the use of the drug Octagam 5% and 10% in various hematological pathologies. The study in the control group involved conditionally healthy donors. Patients with hematological diagnoses were included in the main group, and in some of them they underwent a complex treatment with the use of the drug Octagam. The use of the drug Octagam showed its high clinical efficacy as a drug and allowed to reduce the time of hospitalization, the duration of infectious complications and the timing of their occurrence and the increase in the duration of remission of the main hematological disease.
Keywords: Octagam, Hematological pathologies, Patients with hematological diagnoses, Clinical efficacy
Cite this paper: Feruza Abdulkhamitovna Rizaeva, Khamid Yakubovich Karimov, Study of the Effectiveness of the Use of the Drug Immunoglobulin for Intravenous Administration of "Octagam" as Part of a Complex Treatment of Hematological Diseases in Uzbekistan, American Journal of Medicine and Medical Sciences, Vol. 9 No. 1, 2019, pp. 1-6. doi: 10.5923/j.ajmms.20190901.01.
Article Outline
1. Introduction
- As is known, the immune system of hematological and oncohematological patients is in a depressed state. The exposure of this category of patients to various infectious complications is the subject of a study by various authors. Among the many infectious agents, viruses are of particular importance, among which a special place can be assigned to the parvovirus B19 (PV B19). It is known that parvovirus infection is often associated with the development of aplastic crises, hematological diseases, which are accompanied by inhibition of the hematopoietic and, in particular, erythroid germ, and also reveal an association with the development of hematological diseases [2].The use of standard polychemotherapy in combination with antiviral therapy does not always help to achieve the desired effect. As is known, in individuals with hematologic and hematological diseases, there are often violations and imbalances in the immune system.The use of immunoglobulins in conditions involving immunodeficiencies and immunodeficiencies is one of the effective methods of accompanying therapy in clinical practice [1]. Immunoglobulins for intravenous administration, with immunomodulating purpose, have been used since the 1980s [8]. At about the same time, the first publications appeared highlighting the use of intravenous immunoglobulins in hematology and oncohematology. In particular, J. Fehr (1982) proved the effectiveness of the use of high doses of intravenous immunoglobulins (IVIG) in immune thrombocytopenia (ITP) [4, 21].As you know, each immunoglobulin preparation for intravenous administration has its own characteristics, its own range of advantages and disadvantages. At the same time, immunoglobulin preparations cannot be considered completely interchangeable. Each of them has its own spectrum of possibilities and its own peculiarities related to their purpose. Sometimes it is the right choice of an immunoglobulin drug that ensures the effectiveness and safety of the treatment of infectious viral complications [7, 18], including for hematological diseases [10].It is known that immunoglobulins for intravenous administration, as a rule, are made from blood of at least 1 thousand donors, since only in this case a sufficient number of antibodies to the most diverse antigens will be contained in an immunoglobulin preparation. Drugs of this class have a complex multicomponent mechanism of action. Thus, the presence of opsonizing and neutralizing antibodies, causes the serum bactericidal activity, stimulates phagocytosis, neutralizes superantigens and toxins. Also, immunoglobulins are able to inhibit the development of autoimmune reactions, due to the presence of anti-idiotypic antibodies in their composition, and due to their ability to bind C3 and C4 complement components have the ability to affect the production and activity of various cytokines, including the widely studied tumor necrosis factor (TNF), interleukins-2 and - 6 (IL-2, IL-6) [9].For preparations containing only immunoglobulins G-classes (IgG), in the absence of other classes of antibodies, such as immunoglobulins M и A (IgM and IgA), the content of which cannot exceed 2%, which helps to avoid unwanted adverse reactions, include Octapharma (Vienna, Austria).One of the most well-known, widely used and well-recommended drugs is Octagam (Sandoglobulin®, Novartis, Switzerland or Octagam® 5% or Octapharma, Austria). "Octagam" is a sterile, well-purified, sucrose-free, and ready-to-use intravenous immunoglobulin preparation. Initially, Octagam was used to treat primary and secondary immunodeficiency states and immune thrombocytopenia [9] [17]. Currently, the literature describes the use of the drug “Octagam” for hematological and oncohematological diseases, which has become a common clinical practice [3, 13]. However, questions of the effectiveness of the use of certain hematological diseases may still remain open. Thus, the pharmacoeconomic analysis of individual studies proves the safety and efficacy of using Octagam in hematological diseases by reducing the costs of treating complications and relapses [3]. One of the reasons for the appointment of the drug "Octagam" are infectious processes, especially in the case of an unknown etiology or etiology process [11, 12].According to the literature, the use of intravenous immunoglobulin preparations in hematology is recommended for partial red-cell aplasia, Diamond-Blackfen anemia, autoimmune hemolytic and aplastic anemia. Moreover, in case of idiopathic thrombocytopenic purpura (ITP), as well as various types of leukemia, and in patients after hematopoietic stem cell transplantation and other pathological conditions and syndromes associated with diseases of the blood system, it is recommended even as a first line therapy [6, 3].
2. Main Body
2.1. Purpose of the Study
- Evaluation of tolerability, safety and efficacy of the drug Octagam 5% and 10% for various hematological pathologies.
2.2. Material and Methods of Investigation
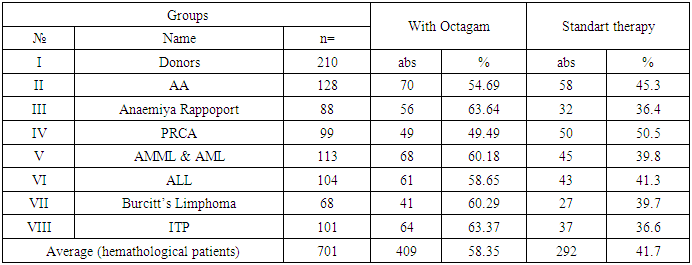
- The study involved a total of 210 conditionally healthy donors considered as a control group. In the main group, 701 patients were involved with a hematological diagnosis, of which 409 patients underwent complex treatment with the use of Octagam, which accounted for 58.3% of the total size of the group, which corresponded, on average, with minor deviations from the proportion of patients receiving therapy drug "Octagam" in each individual group.88 patients were included in group III of patients with Anemia of Rapoport, 56 of whom received therapy with Octagam, and in group IV patients with partial red cell aplasia (PRCA) numbering 99 patients, 49 patients received therapy with Octagam. Among 113 patients with acute myeloid and monoblasts-myeloid leukemias (AML & AMML), Octagam was treated with 68 patients, and out of 104 patients with acute lymphoid leukemiya (ALL) who were involved in the study, 61 patients, and out of 68 patients with Lymphoma Burkitt, 64 patients and 101 patients with ITP, 64 patients, respectively. Therapy was carried out drug "Octagam". In general, among hematological patients in the II-VIII groups, the proportion of patients receiving Octagam was from 49.5% to 63.4%, respectively.Patients were monitored for possible adverse reactions during and after complex treatment, including Octagam (table 1). The so-called adverse drug reactions (ADR) reactions could include nausea, the appearance of transient pain in the lumbar region, dizziness, itching. Also evaluated the severity of these reactions.
|
2.3. Results and Discussion
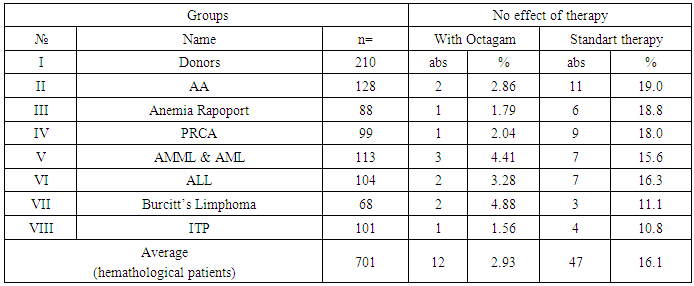
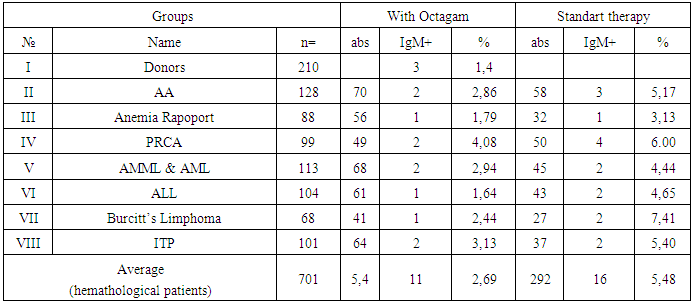
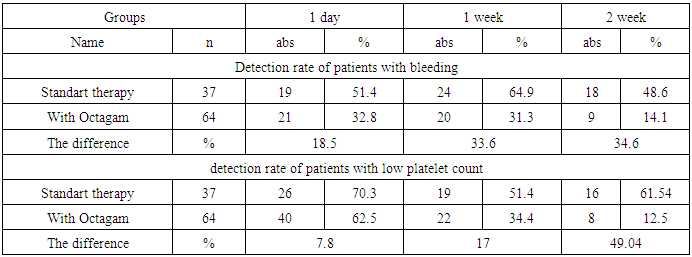
- Of the 104 patients with ALL who received polychemotherapy using the MB-2008 protocol, 76 patients were in the first stage of chemotherapy for PCT, 26 patients in this category received anti-relapse therapy, 69 were supportive, and in 28 patients with ALL, the protocol of PCT treatment was used (II protocol). At the same time, out of 113 patients with AML and AMML who received polychemotherapy using the AML-BFM-2004 protocols, 79 received the protocol for the first time, 63 were in the induction phase, 23 received anti-relapse therapy, 71 were supportive therapies, and 34 patients received the PCT treatment protocol.A total of 21 events or so-called adverse drug reactions (ADR) were recorded, including: nausea (n = 11), the appearance of transient pain in the lumbar region (n = 8), dizziness (n = 4), which had moderate or moderate severity.The number of patients with hematological diseases in whom the use of Octagam was ineffective was 3.0%, of which the proportion of patients with AA, Anemia Rapoport and PRCA was 2.9%, 1.8%, 2.0%, and patients with AML, ALL, Lymphoma Burkitt’s and ITP – 4.4%, 3.3%, 4.9% and 1.6%, respectively. The number of complications was insignificant and the complications were not severe or moderately severe (table 2).
|
|
|
3. Conclusions
- The use of the immunoglobulin preparation for intravenous administration of Octagam showed good tolerability, without the presence of serious side effects and an extremely small number of drug reactions of mild, moderate and moderate severity.The use of the drug "Octagam" showed its high clinical efficacy as a drug that allowed to reduce the time of hospitalization, the duration of infectious complications and the main hematological disease, the timing of the onset and the increase in the duration of remission.
ACKNOWLEDGEMENTS
- The author thanks the scientific adviser and the head of the Department of Molecular Medicine and Cell Technologies of, Scientific Research Institute of Hematology and Blood Transfusion of the Ministry of Health of the Republic of Uzbekistan Hamid Yakubovich Karimov for assistance in the management and organization of the conducted studies.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML