-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2018; 8(2): 355-359
doi:10.5923/j.ajmms.20180812.01

Analysis of the Significance of IIE 105Val Polymorphism of the GSTP1 Gene in the Development of Allergodermatosis in Uzbekistan
Shahnoza Zakirovna Mavlyanova1, Kodirjon Tukhtabaevich Boboev2, Sevara Muradovna Alimova3
1Department of Dermatology, Republican Specialized Scientific and Practical Medical Center of Dermatology and Venereology of the Ministry of Health of the Republic of Uzbekistan, Tashkent, Uzbekistan
2Department of Molecular Medicine and Cell Technologies, Scientific Research Institute of Hematology and Blood Transfusion of the Ministry of Health of the Republic of Uzbekistan, Tashkent, Uzbekistan
3Department of Dermacosmetology, Republican Specialized Scientific and Practical Medical Center of Dermatology and Venereology of the Ministry of Health of the Republic of Uzbekistan, Tashkent, Uzbekistan
Correspondence to: Sevara Muradovna Alimova, Department of Dermacosmetology, Republican Specialized Scientific and Practical Medical Center of Dermatology and Venereology of the Ministry of Health of the Republic of Uzbekistan, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

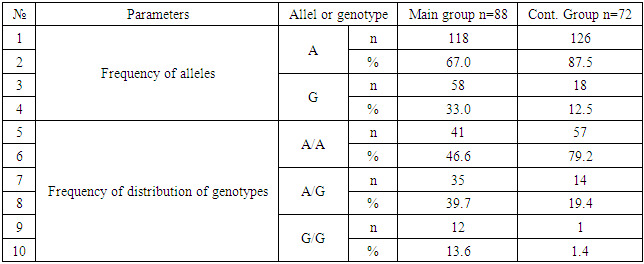
The purpose of research was to establish the role of polymorph variant IIe 105Val gene GSTP1 enzyme biotransformation of xenobiotic in the mechanism of formation and development of allergic skin diseases to substantiate genetic and biochemical criteria for assessing the risk of dermatosis. Thus, allele G and hetero/homozygous genotypes of polymorphism IIe 105 Val of GSTP1 gene are significant markers of increased risk of allergic skin diseases in Uzbekistan (P <0.05). Allele A and the functionally beneficial genotype A/A are reliable protective markers for the development of pathology (χ2=16.5; P<0.05; OR=0.2; 95%CI 0.1186-0.4868).
Keywords: Allergodermatosis, Enzyme biotransformation of xenobiotic, Polymorph variant IIe 105Val gene GSTP1
Cite this paper: Shahnoza Zakirovna Mavlyanova, Kodirjon Tukhtabaevich Boboev, Sevara Muradovna Alimova, Analysis of the Significance of IIE 105Val Polymorphism of the GSTP1 Gene in the Development of Allergodermatosis in Uzbekistan, American Journal of Medicine and Medical Sciences, Vol. 8 No. 2, 2018, pp. 355-359. doi: 10.5923/j.ajmms.20180812.01.
Article Outline
1. Introduction
- The ubiquitous high prevalence of allergic skin diseases in the structure of skin pathology, the tendency to continuing growth, makes it necessary to further study the pathogenetic features of the formation of allergic dermatoses to identify genetic and biochemical markers of predisposition. To date, special attention has been paid to genomic and proteomic studies on the study of genetically biochemical polymorphic systems and the relationship of individual allelic variants of genes with various pathological processes, as well as the intensity of biochemical reactions [A. I. Archakov, 2002, V. M. Govorun, 2003, V. A. Spitsyn, 2006, L. P. Kuzmina, 2010, K. O. Mironov, 2010].It should be noted that in the pathogenesis of allergic diseases, along with the main trigeric factors, so-called modifier genes are involved, the effect of which is largely determined by environmental factors. Among these genes, the genes of glutathione-S-transferase-GST, which encode enzymes of the second phase of biotransformation of xenobiotics, are of particular interest. These enzymes are responsible for biotransformation of incoming chemical, biological agents, medicines. Particular attention is paid to the enzyme of the second phase of detoxification of glutathione-transferase – the product of the GSTP1 gene. The polymorphism of the GSTP1 gene is due to the replacement of nucleotides at positions 313 and 341, which leads to the appearance of three functionally different forms of the enzyme GSTP1 * a, * b, * c.However, the results of studies on these genes are contradictory, which requires further study and refinement of their contribution to the predisposition to the formation and development of allergic dermatoses.
2. Main Body
2.1. The Purpose of Our Research
- Was to establish the role of polymorph variant IIe 105Val gene GSTP1 enzyme biotransformation of xenobiotic in the mechanism of formation and development of allergic skin diseases to substantiate genetic and biochemical criteria for assessing the risk of dermatosis.
2.2. Material and Methods of Study
- The subject and the subject of the study were patients with allergic dermatoses (AlD), DNA samples of patients and healthy donors, glutathione-transferase gene GSTP1 (IIe 105 Val).The study included 88 patients with AlD Disease at the age of 5 to 67 years, observed on the basis of the clinic of dermatology сenter. Of these, 41 are women, 50 are men. The diagnosis in all patients is confirmed by the results of the clinical examination and laboratory tests. All patients were examined, observed and treated in the department of dermatology. Molecular-genetic examination of biomaterials (DNA) was carried out on the basis of the Department of Molecular Medicine and Cell Technologies of the Research Institute of Hematology and Blood Transfusion of the Ministry of Health of the Republic of Uzbekistan.
2.3. Results of the Study
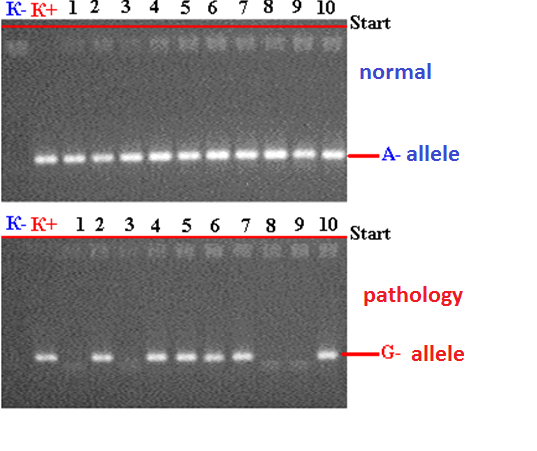
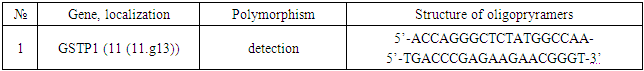
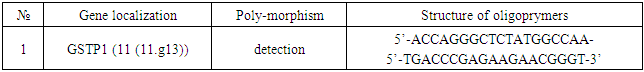
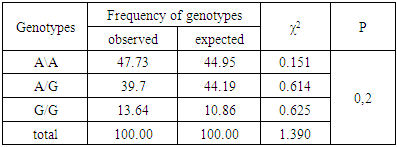
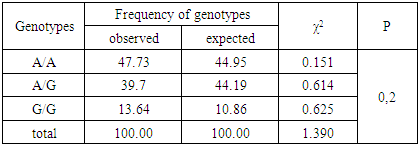
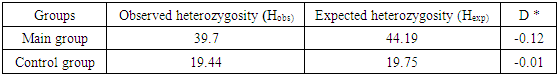
- Among 88 patients with Alder Disease at the age of 14 years were 13, 15-20 years old - 12, 21-30 - 17, 31-40 years - 14, over 40 years - 32 patients. Allergic dermatitis was diagnosed in 88 patients with atopic dermatitis in 49 (55.7%) patients, 28 (31.8%) with urticaria and 11 (12.5%) with allergic dermatitis, respectively. Information on gene sequences and the structure of primers was obtained taking into account the original literature source [5] and GeneBankThe characteristics of the genetic marker and the sequence of synthesized oligopramers are given in Table 1.
|
|
|
|
|
|
3. Conclusions
- Thus, allele G and hetero / homozygous genotypes of polymorphism IIe 105 Val of GSTP1 gene are significant markers of increased risk of allergic skin diseases in Uzbekistan (P <0.05). Allele A and the functionally beneficial genotype A/A are reliable protective markers for the development of pathology (χ2=16.5; P<0.05; OR=0.2; 95%CI 0.1186-0.4868).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML