-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2018; 8(1): 348-354
doi:10.5923/j.ajmms.20180811.10

The Prognostic Value of Mutant Gene P53 in Patients with Osteosarcoma
Polatova D. Sh., Gildieva M. S., Gafur-Akhunov M. A., Abdikarimov Kh. G., Islamov U. F., Urunbaev S. D., Davlatov R. R., Sultanov B. B.
Republican Specialized Practical Scientific Medical Center of Oncology and Radiology of the Ministry of Health of the Republic of Uzbekistan, Tashkent, Uzbekistan
Correspondence to: Polatova D. Sh., Republican Specialized Practical Scientific Medical Center of Oncology and Radiology of the Ministry of Health of the Republic of Uzbekistan, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

It is believed that the main factors determining the prognosis for cancer are the prevalence and degree of differentiation of the tumor. In recent years, more and more attention has been paid to genetic factors that determine the individual risk of developing cancer, and also participate in the processes of carcinogenesis in general. Therefore, the study of molecular-biological tumor markers, which are specific indicators of the high risk of tumor progression and the relationship to treatment efficacy, has acquired clinical significance. Objective. To study the effectiveness of treatment of patients with osteosarcoma depending on the change in expression of the p53 gene. Materials and methods. Under supervision were 221 patients with osteosarcoma. Patients received various types of treatment. Neoadjuvant polychemotherapy (PCT) was performed in 184 (83.2%) patients, of which 131 (71.2%) patients underwent systemic neoadjuvant CT, and 29 (15.7%) patients underwent prolonged intraarterial regional chemotherapy (PIRC). A combination of systemic PCT and PIRC was performed by 24 (13.0%) patients. IHC was performed by avidin-biotin-peroxidase using a standard procedure using primary antibodies (Dako, Novocastra ™) to mp53. Results. Analysis of the obtained data made it possible to establish that expression and overexpression of mtp 53+ occurs in patients with osteosarcoma with a low degree of tumor differentiation (G3) 9 times more often. In patients with stage 3 and 4 clinical stages of osteosarcoma, the positive response of mtp 53+ was 4-fold higher compared to patients with 1-2 stages. Achievement of complete morphological regression during IHC was revealed in 13% of patients, among them in 70.6% of patients the expression of mutant p53 gene was absent. At 15.7% after the use of the PIRC scheme, complete morphological regression was observed, in 87.5% of these patients the mutant p53 gene was also absent. The conclusion. High and average expression of the mutant p53 gene in patients with OS has an associative association with a low degree of tumor differentiation (G3), with stages 3 and 4 of the tumor process, with a large tumor size, low pathomorphism (1 and 2), with a chondroblastic histological variant and osteolytic radiological form. The decrease in the timing of relapses, distant metastases and life expectancy in patients with mild and high expression of the mtp53 gene was found, regardless of the therapy.
Keywords: Osteosarcoma, Polychemotherapy, Mtp53 gene, Survival and risk function
Cite this paper: Polatova D. Sh., Gildieva M. S., Gafur-Akhunov M. A., Abdikarimov Kh. G., Islamov U. F., Urunbaev S. D., Davlatov R. R., Sultanov B. B., The Prognostic Value of Mutant Gene P53 in Patients with Osteosarcoma, American Journal of Medicine and Medical Sciences, Vol. 8 No. 1, 2018, pp. 348-354. doi: 10.5923/j.ajmms.20180811.10.
Article Outline
1. Introduction
- It is known that osteosarcoma is an extremely aggressive malignant tumor prone to rapid growth and hematogenous metastasis. The literature presents a sufficient number of studies aimed at studying the features of bone tissue metabolism in normal and with bone formation in order to identify new potential molecular-biological markers of tumor growth [1]. Many researchers believe that it is the imbalance in the expression of these factors that can be one of the causes of tumor growth. It is believed that they determine the fundamental properties of tumor cells, such as metastasis, invasion, unlimited proliferation, the activity of neoangiogenesis processes, the ability to withstand apoptosis, and their sensitivity to exogenous and endogenous regulators [2].Until now it was believed that the prevalence and degree of differentiation of the tumor are the main factors determining the prognosis for oncological diseases. However, in recent years, increasing attention has been paid to genetic factors that not only determine the individual risk of developing cancer, but are necessary for understanding the processes of carcinogenesis in general. Therefore, the study of molecular-biological tumor markers, considered to be specific indicators of the high risk of tumor progression and significantly correlating with the effectiveness of treatment and the life expectancy of patients, became of great clinical importance [3, 4].It is established that the pathogenesis of many oncopathologies is associated with the inability of transformed cells to undergo apoptosis. One of the mechanisms of apoptosis disorder in tumor cells are mutations in the p53 gene, which interfere with the functioning of the protein it encodes as an inducer of apoptosis. About 50% of human tumors express a mutated form of the p53 gene, which leads to suppression of apoptosis [5, 6, 7, 10]. For an in-depth understanding of the basics of certain stages in the development of tumors, it is necessary to study the role of molecular markers. This is an important factor in terms of diagnosis when studying the activity of malignant growth, in particular in sarcoma of bones [8]. In this regard, the study of the mechanisms of tumor development remains an urgent task of medicine and requires the use of modern methods of research and treatment to address both theoretical and practical issues of oncology. In addition, an important task is to improve the results of treatment by overcoming the resistance of tumor cells against the background of polychemotherapy and the development of new effective pathogenetic therapies [9].
2. The Purpose of the Research
- To study the effectiveness of treatment of patients with osteosarcoma depending on the change in expression of the p53 gene.
3. Materials and Methods
- The research was carried at Republican Specialized Scientific and Practical Medical Center of Oncology and Radiology of the Ministry of Health of the Republic of Uzbekistan from 2005 to 2016. We observed 221 patients with osteosarcoma. Рatients received various types of treatment: 184 (83.2%) patients received non-adjuvant polychemotherapy (PCT), of which 131 (71.2%) patients underwent systemic neoadjuvant CT, 29 (15.7%) patients underwent prolonged intra-arterial regional chemotherapy (PIRC), a combination of systemic PCT and PIRC was performed by 24 (13.0%) patients. In order to obtain adequate results of the study, the groups were composed of patients of comparable age, tumor location, stage of the disease.The diagnostic program of the patients included standard clinical and laboratory tests: objective examination, collection of anamnesis, X-ray examination of the affected bone, chest X-ray, ultrasound of the tumor zone and surrounding tissues, regional lymph nodes, abdominal cavity and retroperitoneal space, to identify remote metastases. All patients underwent cytological examination of the primary tumor and mandatory histological examination of biopsy and postoperative material with an evaluation of the degree of therapeutic pathomorphosis. Also, all patients underwent electrocardiography, study of peripheral blood parameters, immunological examination, and biochemical parameters of liver function, kidney function, MSCT and magnetic resonance imaging, according to indications, a radioisotope study of the bones of the skeleton was performed. In the presence of chronic somatic diseases observed in our patients, consultations were conducted with other specialists (endocrinologist, gynecologist, neuropathologist, cardiologist, etc.).The IHC study was performed on sections from paraffin blocks 3-4 μm thick with avidin-biotin-peroxidase by a standard method using primary antibodies (Dako, Novocastra ™) to mp53. Biotinylated antibodies to murine and rabbit immunoglobulins ("Dako", Novocastra ™) were used as secondary antibodies. Avidin-Biotin Complex (ABC) Method is standard IHC method and one of widely used technique for immunhistochemical staining. Avidin, a large glycoprotein, can be labeled with peroxidase or fluorescein and has a very high affinity for biotin. Biotin, a low molecular weight vitamin, can be conjugated to a variety of biological molecules such as antibodies. The technique involves three layers. The first layer is unlabeled primary antibody. The second layer is biotinylated secondary antibody. The third layer is a complex of avidin-biotin peroxidase. The peroxidase is then developed by the DAB or other substrate to produce different colorimetric end products [11]. A specific amount of positively stained tumor cells expressing mtp53 was evaluated. A positive result was the presence of a specific high coding intensity of more than 10% of the nuclei when a mutated suppressor gene mtp53 was detected.Immunohistochemical staining makes it possible to obtain a unique material for evaluating the biological potential of tumor cells: the rate of growth, the prognosis of the course of the tumor process, the response to hormonal treatment and specific immunotherapy.We analyzed the treatment of patients with osteosarcoma between 2005 and 2016. 184 (83.2%) patients received non-adjuvant polychemotherapy (PCT), of which 131 (71.2%) patients underwent systemic neoadjuvant CT, 29 (15.7%) patients underwent prolonged intra-arterial regional chemotherapy (PIRC), a combination of systemic PCT and PIRC were performed by 24 (13.0%) patients. Patients received different schemes of PCT: 74 patients (56.5%) were treated according to the CAP scheme, EAP 10 (7.6%), VCAP - 17 (12.9%), MAP - 10 (7.6%) and AP - 20 (15.3%). Diagrams of the PIRC were as follows: 43 patients (81.1%), AP-4 (7.5%), MAP-4 (7.5%) and VCAP-2 (5.7%) were treated according to the PIRC scheme. The number of courses varied and depended on the response to treatment, on average, with systemic PCT, was 3.06 ± 0.16, with a PIRC of 2.81 ± 0.18. The interval between the cycles was 21 days.Statistical processing of the clinical material was carried out using the "EXCEL" and "STATGRAFICS" software package (StatSoftInc., USA). The reliability of the revealed differences in the analysis of quantitative traits was estimated by the Student-Fisher criterion. In the study of patient survival, the method of constructing survival tables Kaplan-Mayer (1958), recommended for use by the International Anti-Cancer Union (IACU), was used. For statistically significant changes, the level of confidence was P < 0.05.
4. Results and Discussion
- We have studied the relationship of the expression of the mutant p53 gene (mtp 53) with the age and sex of patients with osteosarcoma. The analysis showed that the expression of the mutant p53 + gene (more than 10% of the cells with intense color) and its absence of mtp53- (less than 10% of the cells with weak color) in tumor cells do not have an association with the age and gender of patients.Also, the analysis of the obtained data made it possible to establish that expression and overexpression of mtp 53+ occurs in patients with low grade tumor necrosis (G3) 9 times more often (90.1% of patients) than with high (G1 - 9.9%). The tendency of the connection of positive expression of mtp53 with the prevalence of the process (T2) is traced. However, with 3 to 4 clinical stages of osteosarcoma, the association is more reliable, since the frequency of occurrence of patients with a positive response of this marker was more than 4 times higher than in patients with 1-2 stages (70.5% and 85.7% compared with 20.0% and 47.8% p < 0.05).In patients with a large tumor volume (more than 500 cm3), positive expression of mtp53 was 1.5 times more common than in patients with a smaller tumor size (260 cm3). Chondroblastic histological variant (41.4%) and osteolytic radiological form (43%), which occur with a more malignant phenotype, were also more frequent in patients with positive expression of mtp53.The increase in nuclear accumulation of mutant p53 protein in tumors of osteosarcoma patients adversely affects the kinetic processes in tumor cells and determines the acquisition of additional signs of facilitating metastasis.It is known that a low level of spontaneous or induced apoptosis of tumor cells can be the basis for developing resistance to antitumor therapy. Thus, in systemic chemotherapy (Table 1), a tumor reduction of 75% was observed only in 38.9% of patients with positive mtp53 + gene expression, compared with 61.1% of patients without this mutation. Pathomorphosis of 1 and 2 degrees was 2.65 times more frequent in patients with positive expression of the mtp53 + gene, and grade 3 - 4 in 2.9 times were more frequent in patients with no such mutation.A complete regression of osteosarcoma was observed in 21.4% (28/131) of patients, with more than half (75.0%) having a negative mtp53 phenotype. A partial regression of the tumor was observed in 63.4% of the examined patients, and there was no correlation with the presence of mtp53 gene expression among them. 41.5% of these patients had high and medium expression of the mutant gene. In 32.8% of patients, stabilization of the tumor process was registered, with 74.4% expressing and overexpressing the mtp53 gene. Progression of the tumor process was established in 32.1% (42/131) of patients. Among them, 76.2% had a mutant form of the p53 gene (Table 1).
|
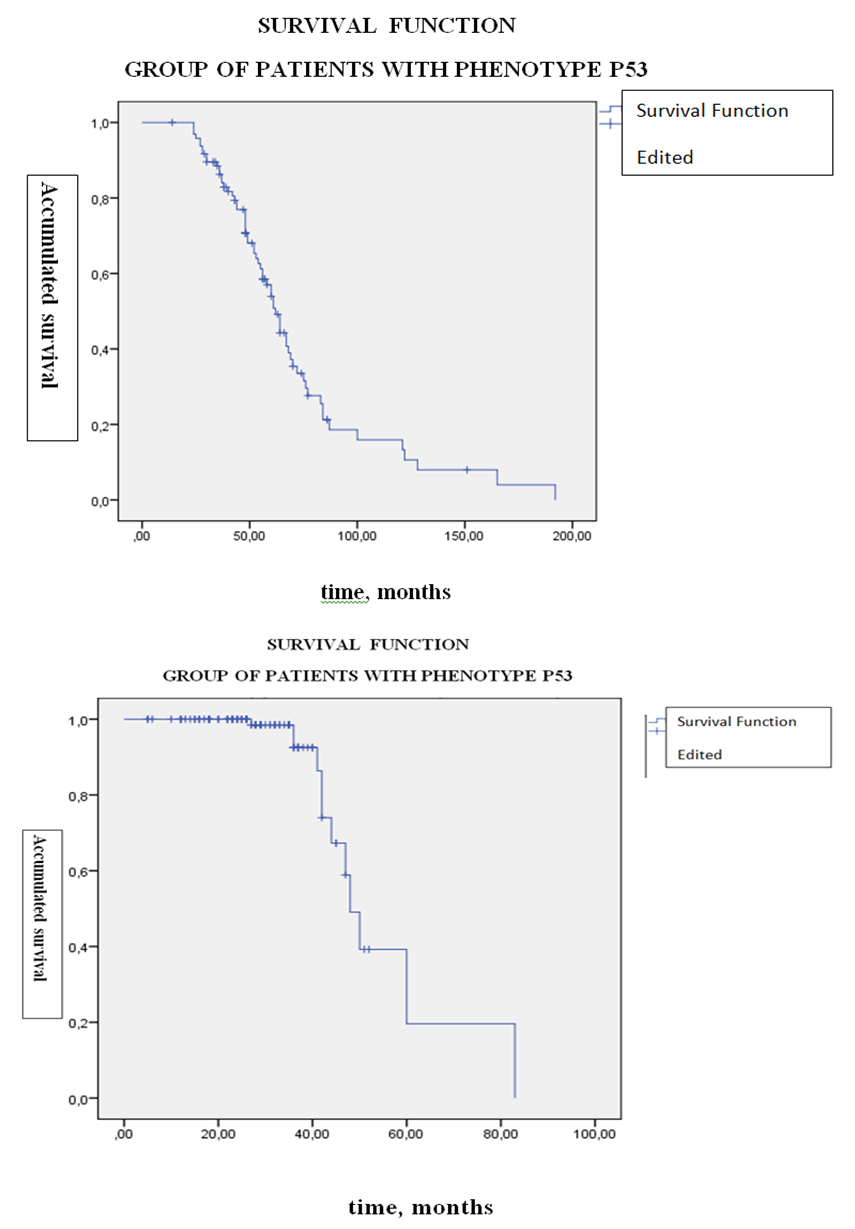
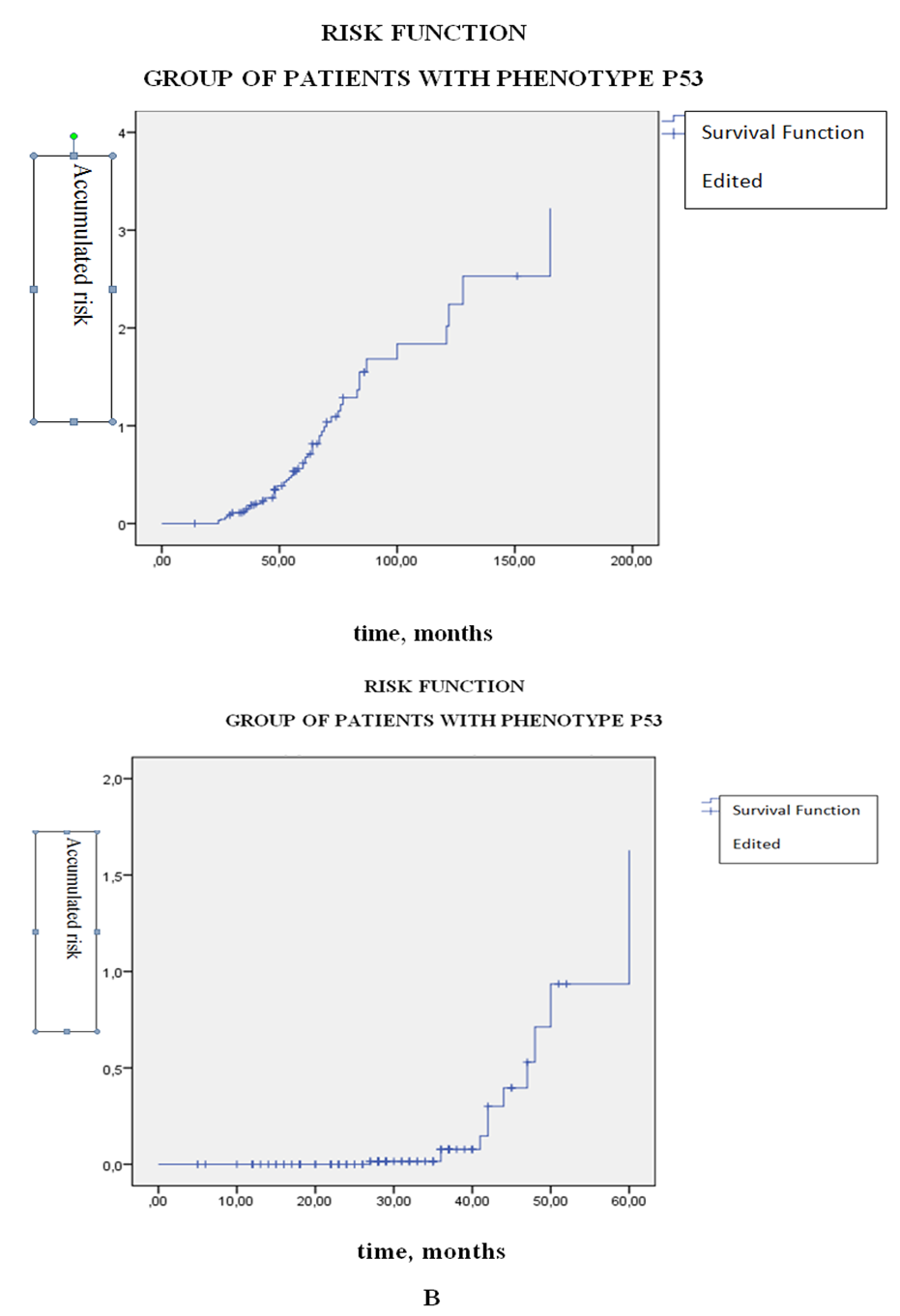 | Figure 2. Life time graph and risk function (Kaplan-Meier method) from the group of patients with osteogenic sarcoma (n = 117) having the phenotype p53 +. A - survival function; B is a risk function |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML