-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2018; 8(1): 324-328
doi:10.5923/j.ajmms.20180811.05

Value of the Polymorphism rs1800471 TGFB1 Gene in the Formation of a Predisposition to Duodenal Ulcer and Chronic Gastritis
Alisher Alijonovich Yariyev, Khamid Yakubovich Karimov, Kodirjon Tukhtabaevich Boboev
Department of Molecular Medicine and Cell Technologies, Scientific Research Institute of Hematology and Blood Transfusion of the Ministry of Health of the Republic of Uzbekistan, Tashkent, Uzbekistan
Correspondence to: Alisher Alijonovich Yariyev, Department of Molecular Medicine and Cell Technologies, Scientific Research Institute of Hematology and Blood Transfusion of the Ministry of Health of the Republic of Uzbekistan, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

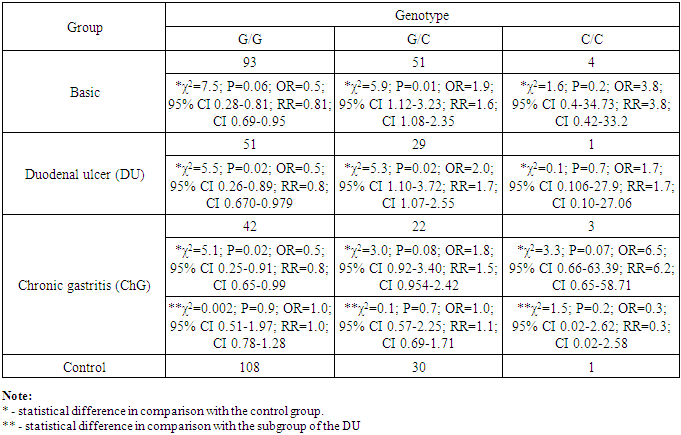
Conducted the detection of genetic polymorphism rs1800471 of TGFb1 gene in unrelated patients of Uzbek nationality with clinically and endoscopically established gastroduodenal diseases, including in patients with diagnosed complicated forms of duodenal ulcer (DU) and chronic gastritis (ChG). It is determined that the carriage of the heterozygous G/C genotype of the TGFb1 gene (rs1800471) is associated with a high risk of development of DU and ChG. It is also revealed that the homozygous genotype G/G possesses a protective anti-inflammatory role in the pathogenesis of ulcerative and inflammatory lesions of the gastric mucosa and duodenum. The burden of unfavorable homozygous genotype C/S in 1.7 and 6.5 times statistically increases the risk of development of DU and ChG, respectively. There were no statistically significant differences between DU and ChG.
Keywords: Polymorphism rs1800471 of TGFb1 gene, Duodenal ulcer (DU), Chronic gastritis (СhG), Genotype, Protective
Cite this paper: Alisher Alijonovich Yariyev, Khamid Yakubovich Karimov, Kodirjon Tukhtabaevich Boboev, Value of the Polymorphism rs1800471 TGFB1 Gene in the Formation of a Predisposition to Duodenal Ulcer and Chronic Gastritis, American Journal of Medicine and Medical Sciences, Vol. 8 No. 1, 2018, pp. 324-328. doi: 10.5923/j.ajmms.20180811.05.
Article Outline
1. Introduction
- As is known, the superfamily of the transforming growth factor TGF-β (transforming growth factor beta) refers to multifunctional peptides from the cytokine group that controls proliferation, differentiation, and other functions in many cell types. They play a fundamental role in the regulation of basic biological processes, such as growth, development, maintenance of tissue homeostasis and the functioning of the immune system. TGF-β is considered to be a cytokine of systemic action, since this peptide and its specific receptors have been identified in almost all cell types. [1, 2]. Among the known growth factors, cytokine TGF-β1 is considered the main mediator of fibrogenesis. [3, 4]. In addition to the function of the fibroblast inducer, it is currently recognized as a multifunctional cell growth factor. In particular, TGF-β1 inhibits cell growth by inhibition of the G1 phase of the cell cycle and stimulates apoptosis in various cells [5]. Increased expression of the TGF-β1 gene is associated with pathological conditions due to the processes of fibrosis and scarring [6].TGF-β1 belongs to the group of anti-inflammatory cytokines that inhibits such inflammatory processes modulated by IL-1 and TNF-α, such as homing, cell adhesion, chemotaxis. TGF-β1 inhibits the proliferation of T and B-lymphocytes, inhibits the activity of macrophages and natural killers [7]. Normally, TGF-β1 regulates the growth of cells of the gastric mucosa, in particular the cells of the pit epithelium [10, 8]. TGF-β sequentially activating the proteins of apaptosis (Smad, Bim) and caspase 9, is involved in the physiological regeneration of the gastric mucosa.Recent years in scientific literature discuss the role of polymorphism 915G> C (Arg/Pro, rs1800471) gene TGFB1 in the development of gastroduodenal diseases. However, the role of the genotypic variants of this gene in the formation and development of duodenal ulcer (DU) and chronic gastrit (ChG) has not been studied sufficiently.
2. Main Body
2.1. Purpose of the Study
- Genomic typing of polymorphic region rs1800471 of TGFB1 gene in patients with gastroduodenal diseases and in relatively healthy donors of Uzbek nationality with the subsequent analysis of the possible association of this polymorphism with development of DU and ChG.To study the frequency distribution of the genotypic variants of the polymorphism rs1800471 of the TGFB1 gene among patients with gastroduodenal diseases and to assess their role in the development and characteristics of the clinical course of DU and ChG.
2.2. Material and Methods of Investigation
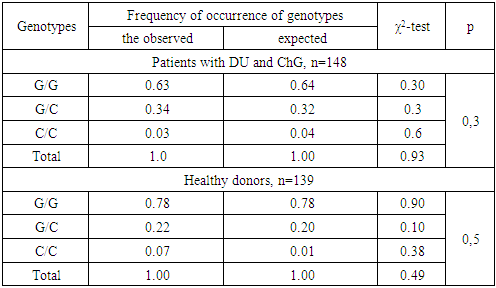
- Detection of genetic polymorphism rs1800471 of TGFb1 gene was performed in 148 unrelated patients of Uzbek nationality with clinically and endoscopically established gastroduodenal diseases, including 81 patients diagnosed with complicated forms of DU and in 67 patients with ChG. Patients with complicated forms of DU were hospitalized at the Republican Scientific Center for Emergency Medical Care, and those with ChG were in the 1st Clinic of the Tashkent Medical Academy.The control group consisted of 139 healthy unrelated persons who had no history of gastrointestinal pathology of the Uzbek nationality, corresponding to the sex and age of the group of patients with gastroduodenal pathology (p> 0.05). All patients or their relatives, as well as individuals of the control group received informed consent to conducting research.The polymorphism rs1800471 of the TGFB1 gene was tested on a programmable thermal cycler from Applied Biosystems, 2720 (USA), using the test systems of the company Liteh (Russia), according to the manufacturer's instructions.Specificity and number of amplified fragments were checked by agarose gel electrophoresis (Figure 1).
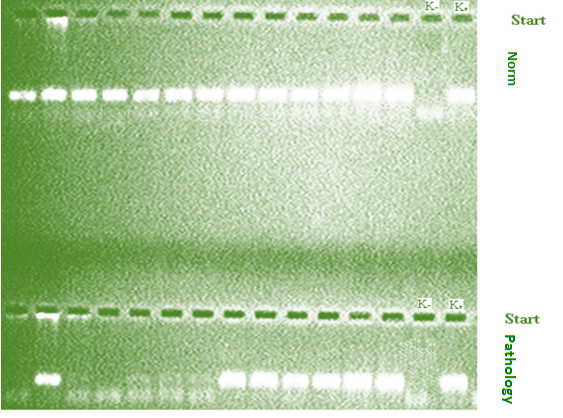 | Figure 1. Electrophoregram of detection of rs1800471 polymorphism of TGFB1 gene |
2.3. Results and Discussion
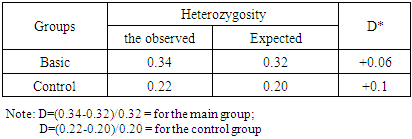
- In patients with gastroduodenal diseases, there is a significant increase in the proportion of carriers of the unfavorable Pro (C) allele from 11.5 to 19.9% (χ2 = 7.6; P = 0.006; OR = 1.9; 95% CI 1.20-3.04). Approximately the same values (x2 = 0.1, P = 0.7, OR = 0.9, 95% CI 0.50-1.58) of the carrier of the Pro (C) allele were noted both in the subgroup of patients with DU (19.1%) and in subgroup of patients with ChG (20.9%), which also differ significantly (χ2 = 4.8, P = 0.03, OR = 1.8, 95% CI 1.06-3.11 and χ2 = 6.4, P = 0.01, OR = 2.0, 95% CI 1.16-3.54, respectively) from the similar parameter of the control group of healthy individuals (11.5%) (Figure 2 and Table 1), which indicates the association of this allele with an increased risk of gastroduodenal diseases.
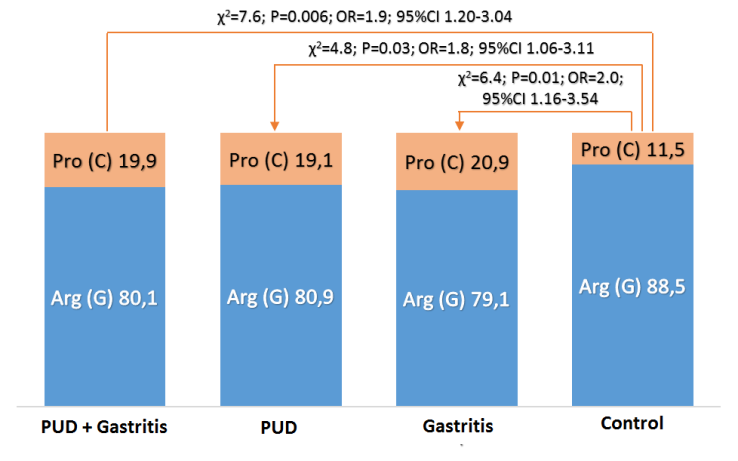 | Figure 2. Frequency of alleles of polymorphism rs1800471 of TGFb1 gene in patients with gastroduodenal ptology and relatively healthy donors |
|
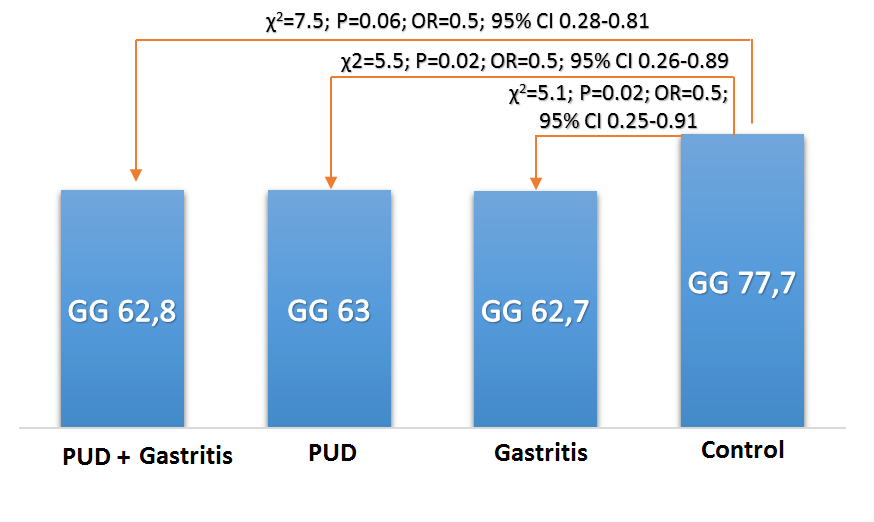 | Figure 3. Frequency of occurrence (%) of G/G genotype rs1800471 of TGFb1 gene in patients with gastroduodenal diseases and conditionally healthy persons of Uzbek nationality |
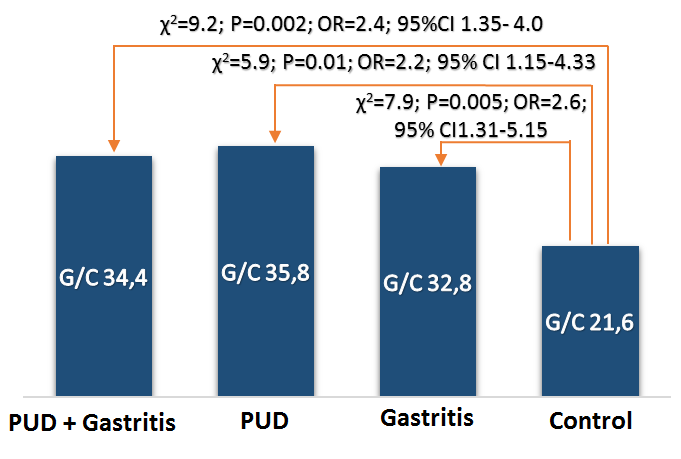 | Figure 4. The frequency of occurrence (%) of the genotype rs1800471 of the gene TGFb1 in patients with gastroduodenal diseases and conditionally healthy persons of Uzbek nationality |
|
|
|
3. Conclusions
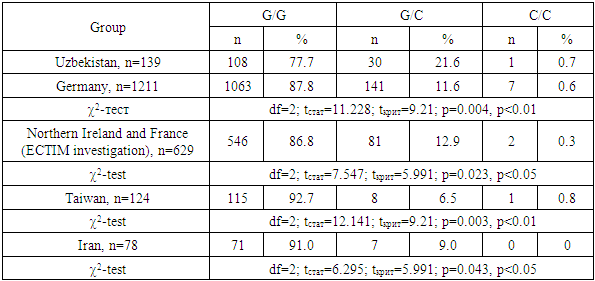
- The carrier of the heterozygous G/C genotype of the TGFb1 gene (rs1800471) is associated with a high risk of development of DU and ChG. The homozygous genotype G/G possesses a protective anti-inflammatory role in the pathogenesis of ulcerative and inflammatory lesions of the gastric mucosa and duodenum. Relatively healthy people of Uzbek nationality are 2-3 times more likely to have carriers of genotypes G/C and C/C in comparison with different populations of Europoid and Asians. This new information, obtained for the first time from the population of Central Asia, complements the database reflecting the differences in the nature of the adhesion of polymorphisms of the TGFB1 gene in different populations.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML