-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2018; 8(1): 312-317
doi:10.5923/j.ajmms.20180811.03

Surgical Treatment of Cardiac Echinococcosis
F. G. Nazirov, M. M. Zufarov, Kh. A. Abdumadjidov, M. M. Akbarov, Sh. N. Khudaybergenov, Kh. J. Buranov
«Republican Specialized Research Centre of Surgery named after academician V.Vakhidov» state unitary enterprise, Tashkent, Uzbekistan
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The results of diagnostic methods and surgical treatment of 56 patients with cardiac echinococcosis have been analyzed. The patients were divided into 2 groups subject to the nature of the lesion: the 1st group – 25 patients with isolated echinococcosis of heart/pericardium, the 2nd group – 31 patients with combined cardiac echinococcosis and the other target organs (lungs or liver). A basic diagnostic technique was echocardiogram and MRI. In 64.2% of cases the patients were performed surgery in the conditions of cardiopulmonary bypass; in 35.7% - without cardiopulmonary bypass. Postoperative lethality made up 8.9%. Spontaneous perforation and anaphylactic shock were observed in 3.5% of cases. Rhythm disturbance was mostly observed as a non-fatal complication.
Keywords: Cardiac echinococcosis, Cardiopulmonary bypass, Surgical treatment
Cite this paper: F. G. Nazirov, M. M. Zufarov, Kh. A. Abdumadjidov, M. M. Akbarov, Sh. N. Khudaybergenov, Kh. J. Buranov, Surgical Treatment of Cardiac Echinococcosis, American Journal of Medicine and Medical Sciences, Vol. 8 No. 1, 2018, pp. 312-317. doi: 10.5923/j.ajmms.20180811.03.
1. Introduction
- Cardiac echinococcosis is a severe zooanthroponosious disease which is characterized by long-duration chronic course, severe organic and systemic pathology, leads to disability and often to the patient’s death [3, 4, 6, 7, 11]. Currently echinococcosis remains a serious social and medical issue in many countries, especially in the endemic regions [33]. It is necessary to point that there is a tendency of increasing patients quantity in the developed countries of Europe and in the USA. This fact, probably, is connected with the growth of emigrants quantity suffering from hydatid disease [33].The Central Asia is the region where a morbidity frequency reaches high digits making up approximately 9 people to 100000 of population [33].In spite of concrete progress of medicamental and surgical treatment of cardiac echinococcosis it still remains a serious medical-social issue. It should be mentioned that there are very few researches on cardiac echinococcosis surgery [6-18]. A small number of publications and clinical observations on diagnostics and surgical treatment testifies an insufficient information awareness of clinicians about diagnostic features and facilities of surgical treatment. There are no opinions agreement in the literature about surgical treatment tactics for cardiac echinococcosis and the issues of simultaneous surgeries in the combined hepatocardial and cardiopulmonary hydatid still remain open [26, 30, 33].
2. Goal
- To analyze and to estimate surgical treatment results of the patients with cardiac echinococcosis.
3. Materials and Methods
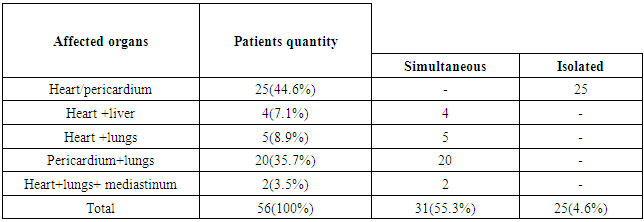
- 56 patients were performed surgery associated with cardiac echinococcosis at the Republican Specialized Research Centre of Surgery named after academician V.Vakhidov between 1994 and 2017. All the patients were divided into 2 groups subject to the nature of parasitogenic lesion: the 1st group – 25(44.6%) patients with isolated cardiopericardiac echinococcosis; the 2nd group – 31(55.3%) patients with combined echinococcosis of heart/pericardium and target organs (liver, lungs, mediastinum).The age of the patients ranged from 7 to 64 years (mean age 26.7 ± 2.7 years) There were 27(48.2%) males and 29 (51.7%) females. The diagnostics of heart parasitogenic lesion was performed by the following investigations: chest X-ray (Fig.3), transthoracal (TT) and trans-esophageal (TE) echocardiography (ECG) (Fig.1; Fig.2), multi-slice computer tomography (MSCT) and coronarography.
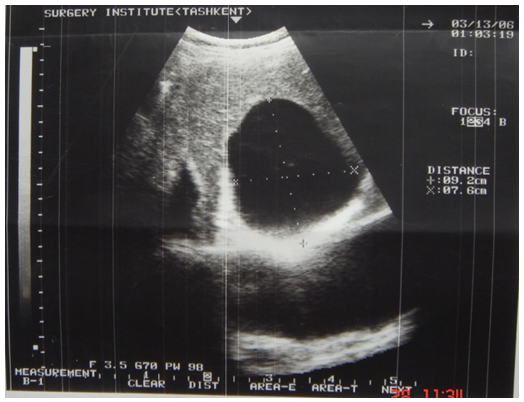 | Figure 1. ECG of LV hydatid |
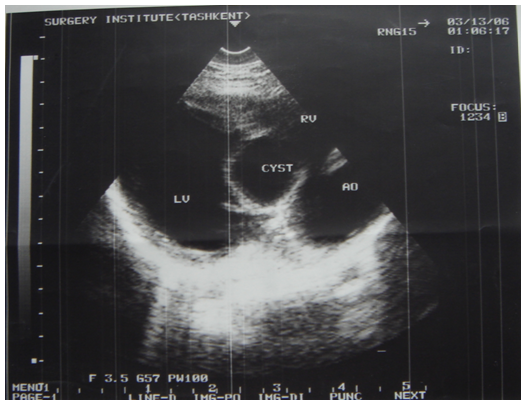 | Figure 2. ECG of interventricular septum hydatid |
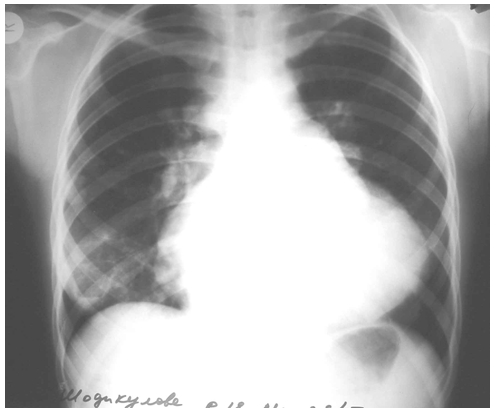 | Figure 3. X-ray pattern of LV hydatid |
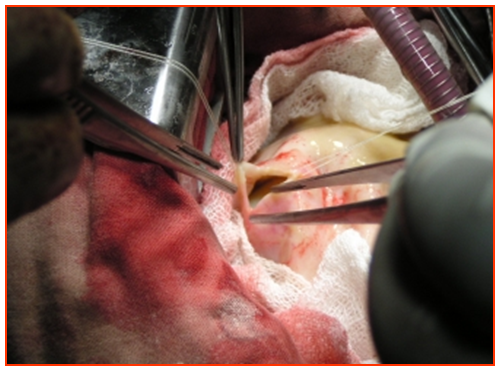 | Figure 4. Cystotomy |
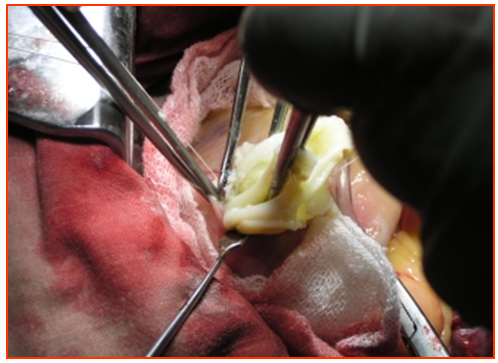 | Figure 5. Removal of the chitin |
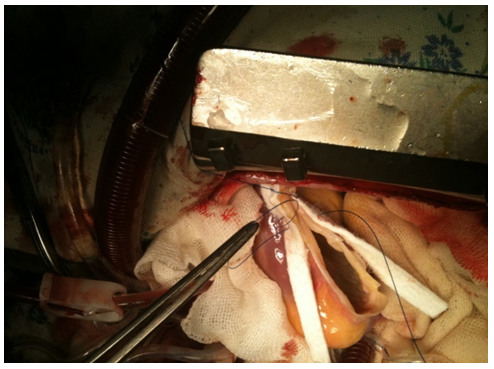 | Figure 6. Closure |
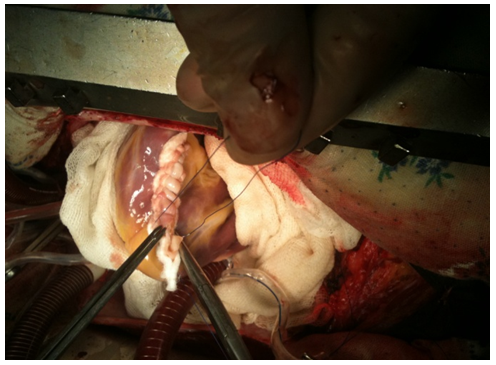 | Figure 7. Final view |
|
4. Results
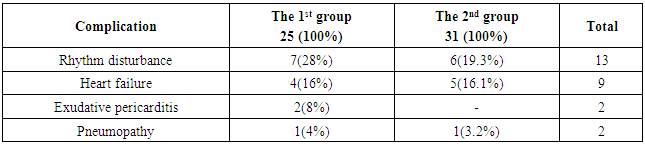
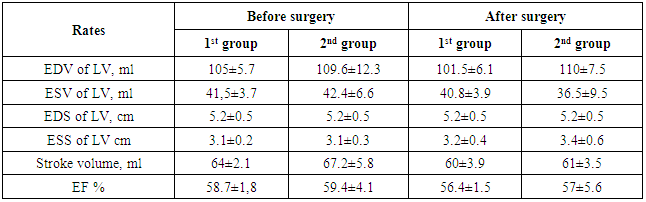
- General postoperative lethality in the groups made up 5 (8.9%) patients. 2 patients from the group with isolated cardiopericardiac echinococcosis group died in the early postoperative group. In one of them we revealed a critical stenosis of anterior interventricular branch (AIVB). The patient was performed simultaneous surgery – EE from the left ventricle and coronary artery bypass grafting (CABG) of AIVB in the conditions of CPB and cardioplegia. The early postoperative period was complicated by hemorrhage: we performed resternotomy, sanation and hemostasis. The patient died due to multiple organ failure. In the second case the lethal outcome was caused by the rupture of the left ventricle wall after the removal of intramurally located cyst.In the group with combined echinococcosis of heart/pericardium and target organs (liver, lungs, mediastinum) the lethality made up 3 (5.3%). In one case the reason of the lethal outcome was an injury of anterior interventricular artery during the removal of hydatid from interventricular septum. Perforations were the fatal complications which had occured in 2 (3.5%) patients during sternotomy. Those patients had perforation of stressed hydatid of the heart right parts to the right ventricle cavity with the development of severe anaphylactic shock which led to the fatal outcome.There was no relapse of the disease during 5 years. Anthelminthic therapy was conducted according to the recommendations.Among non-fatal complications we mostly observed ventricular rhythm disturbance which were registered in 7 (28%) patients with isolated cardiopericardiac echinococcosis and in 6 (19.3%) patients with combined echinococcosis of heart/pericardium and target organs. Heart failure in the patients with isolated cardiopericardiac echinococcosis (the 1st group) was observed in 4 (16%) cases, in the group with combined echinococcosis of heart/pericardium and target organs (the 2nd group) – in 5 (16,1%) patients (Table.2). In 2 (8%) patients of the 1st group we observed a clinical presentation of compressive exudative pericarditis and they were performed pericardium cavity drainage. 2 patients (1 in each group) had pneumopathy. There were no complications connected with abdominal and pleural cavities.
|
|
5. Discussion
- Cardiac echinococcosis is rare parasitogenic lesion and its occurrence frequency does not exceed 2% [1-26, 32-37]. Clinical presentation of cardiac echinococcosis is not specific and depends on the sizes and localization, their relations with neighbouring cardiac structures [1-6, 11-18]. There are some data in the literature about the patients with cardiac echinococcosis taken to the hospitals with clinical presentation of coronary blood flow disturbance [33]. We revealed 22.7% cases of myocardial ischemia by ECG in preoperational period and their signs disappeared after surgery. Only in one case we performed CABG due to the critical stenosis of anterior interventricular artery. In the other observations the ischemia was conditioned by the compression of neighbouring coronary vessels of the stressed hydatid and had a transient nature.In our cohort of patients a combination of cardiac echinococcosis and target organs was observed in 55.3% of cases.According to the literary data a spontaneous perforation of hydatid into pericardium cavity or to the cardial cavity with the development of embolic syndrome and anaphylactic shock is observed in 7-15% of cases [2, 5, 27, 29, 31, 32, 37, 38]. Some authors describe the cases of hydatids perforation during cardiopulmonary resuscitation. In our investigation 2 (3.5%) patients died due to the hydatids perforation in the cardial cavity.A special place in the intravital diagnostics of cardiac echinococcosis belongs to echocardiography [19, 32, 36]. We perform echocardiography and MSCT in all patients – it allows not only to detect hydatids but to detail their topographic localization, size and relation with coronary vessels.Surgical treatment is a method of choice for cardiac echinococcosis by the world literary data [6, 11, 15, 20, 35]. Some authors prefer “off-pump” surgeries from thoracotomic approach, especially for pericardium echinococcosis. This method is interfaced with a number of complications such as: dissemination of hydatid content, a probability of neighbouring coronary vessels accidental taking to the suture during cardiac cycle, hydatid perforation during parasite’s bed handling, the risk of aeroembolism and non-radical removal with a high risk of disease relapse.Analyzing the literature it has been determined that each sixth patient dies due to the hemorrhage during the operative treatment, especially if it is performed “off-pump”. That is why some authors being concerned about the occurrence of complications (hemorrhage, myocardium rupture and etc.) consider performing EE in the conditions of CPB and cardioplegia (CP) as the most reasonable method [11, 21, 26, 33]. We also think that performing EE in the conditions of CPB and CP on immobilized heart is rational and reasonable surgical method. Unfortunately, there is no a unified point of view in the literature concerning surgical treatment tactics of combined cardiac echinococcosis and target organs. Kabbani S.S. et al. [20] in their 8 observations first performed EE from the heart and 3-6 months later – from the liver and the lungs.The up-to-date level of cardio-anesthesiology and cardiac surgery allows to perform successful simultaneous operations in the heart and target organs at combined cardiac echinococcosis. In N.O.Travin’s [33] opinion, at the echinococcosis of pericardium and lungs the simultaneous surgeries are possible due to the hydatids location in the same anatomical cavity. Our investigation results testify the possibility of simultaneous surgeries even at the parasitic cyst’s location simultaneously in the different anatomical cavities (thoracic and abdominal).R. Parvizi et al. [26] offers to undergo a simultaneous surgery at the combined echinococcosis of heart and liver – he used a subcostal approach for hepatic hydatid removal and longitudinal sternotomy – for cardiac hydatid. The lacks of this method are a high traumatism of subcostal approach (transection of all groups of anterior abdominal muscles), a high frequency of postoperative hernias development and deformation of the anterior abdominal wall.A hospital lethality after surgical treatment has not been sufficiently presented in the literature because in the majority of observations they speak about single surgeries. According to the literary data the postoperative lethality made up 5-28% [11, 21, 26, 33]. The postoperative lethality in our cohort of patients made up 8.9%. In 2 patients during 5 years of observation we registered the relapse of the disease. Those patients were successfully re-operated.There is still no unity of opinions for optimal strategy of cardiac echinococcosis medicamental treatment. There are some reports about positive results of conservative treatment with Albendazole [10]. The other authors reduce the area of chemotherapy use without surgical intervention only for the patients with inoperable variants or for the patients who refused from the surgery. The third ones perform cardiac surgery after chemotherapy and also recommend to conduct therapy after the surgery. The fourth ones consider that treatment with Albendazole is deadly dangerous and absolutely contraindicative due to the softening of parasite wall during the treatment and a multiple increasing of hydatid rupture. And at last, the fifth ones point a low efficiency of the chemotherapy even after the hydatids removal. We use the chemotherapy only in the postoperative period for the prevention of the disease relapse.
6. Conclusions
- Transthoracal echocardiography is a screening method for the diagnostics of cardiac echinococcosis. In 55.3% of cases we observed a combination of cardiac echinococcosis and target organs (lungs and liver). It is reasonable to perform echinococcectomy in the conditions of cardiopulmonary bypass and cardioplegia. It is possible to perform a simultaneous surgery at hepatocardiac and cardiopulmonary combined echinococcosis.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML