-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2018; 8(0): 251-258
doi:10.5923/j.ajmms.20180810.01

The Effects of Acute Exercise on Arterial Endothelial Diameter: A Meta-Analysis
José M. Briceño-Torres1, José Moncada-Jiménez1, 2
1School of Physical Education and Sports, University of Costa Rica, Costa Rica
2Human Movement Sciences Research Center – CIMOHU –, University of Costa Rica, Costa Rica
Correspondence to: José Moncada-Jiménez, School of Physical Education and Sports, University of Costa Rica, Costa Rica.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

There is abundant literature regarding the chronic effects of different exercise modalities on arterial diameter and endothelial function; however, there is a dearth of information related to the acute effects. We investigated the acute effects of different exercise modalities on arterial endothelium diameter as measured by flow-mediated dilation (FMD). An electronic search was performed in electronic databases using the following word combinations: “acute exercise”, “FMD”, “endothelial function”, “exercise”, “vascular endothelium”, and “flow-mediated dilation”. The inclusion criteria were experimental studies with single session treatment, studies in healthy human adults, English language publications, and studies reporting pre- and post- flow-FMD measures. The random effects model was used to calculate effect sizes (ES). We included 13 studies (43 effect sizes), representing 378 participants (245 men and 133 women). Twelve studies had poor and very poor quality. Aerobic exercise showed a small effect (ES = 0.27, CI95% = -0.08, 0.62, p = 0.11), resistance training a trivial effect (ES = -0.15, CI95% = -0.76, 0.46, p = 0.25), and interval exercise a small effect (ES = 0.23, CI95% = -0.82, 1.27, p = 0.15) on arterial diameter. In conclusion, aerobic, resistance, and interval training exercises did not elicit a positive arterial diameter acute response. These findings are influenced by the poor methodological quality of the literature.
Keywords: Vascular health, Flow-mediated dilation, Endothelium, Meta-analysis, Acute exercise
Cite this paper: José M. Briceño-Torres, José Moncada-Jiménez, The Effects of Acute Exercise on Arterial Endothelial Diameter: A Meta-Analysis, American Journal of Medicine and Medical Sciences, Vol. 8 No. 0, 2018, pp. 251-258. doi: 10.5923/j.ajmms.20180810.01.
Article Outline
1. Introduction
- Several statements, guidelines and recommendations have been released to the general population regarding the quantity of aerobic and resistance training exercise necessary to achieve a healthy lifestyle [1-7]. However, sedentary behaviour still contributes to the current obesity pandemic and it is associated to several cardiovascular risk factors, including atherosclerosis, a disease related to endothelial dysfunction. There is evidence showing that endothelial function changes according to physical activity level, gender and age [8]. The flow-mediated dilation (FMD) is a technique used to evaluate endothelial function is. This technique underlies a process mediated by nitric oxide (NO) synthesis and bioavailability [9]. It is known that NO stimulates vasodilation in blood vessels; therefore, FMD is considered a good estimate of blood vessel’s health [10, 11].Blood vessel hardening contributes to increased peripheral arterial resistance, as well as increased pressure and ventricular afterload; all of these are cardiovascular risk factors that increase mortality in the population [10]. Aerobic exercise has been widely recommended to prevent these diseases [1]; however, little is known about the potential benefits of acute resistance training exercise on endothelial function. The only evidence found wast a report published by Collier, Diggle, Heffernan, Kelly, Tobin and Fernhall [12], who investigated the acute effects of aerobic and resistance training exercises in healthy young men using the FMD technique to evaluate peripheral arterial resistance. Resistance training exercise increased peripheral arterial resistance more than aerobic exercise did. It is suggested that there could be a compensatory vascular effect when performing resistance training, since there was a higher reactive hyperemia compared to aerobic exercise.According to Cohen [13], the effect size (ES) is a measure of the magnitude of an intervention on a dependent variable. This measure is used in meta-analysis and is considered trivial (ES = 0-0.19), small (ES = 0.20-0.49), medium (ES = 0.50-0.79) and large (ES ≥ 0.80). Recently, two meta-analysis reported on the effects of exercise on endothelial function and arterial diameter [14, 15]. Black, et al. [15], studied whether differences existed on arterial diameter among athletes participating in aerobic, resistance and concurrent exercises. Twenty articles were included in the analysis (aerobic = 7, resistance = 4, concurrent = 9); however, the analysis was performed only with aerobic and concurrent sports given the small sample of resistance training studies. The greatest effect on brachial artery diameter was found for aerobic training (ES = 1.84, 95% confidence interval [CI95%] = 0.59, 3.09, p < 0.01). Ashor, et al. [14], designed a meta-analysis to answer two questions, a) what is the effect of resistance, aerobic and concurrent exercises on endothelial function, and, b) what type of exercise and participant’s characteristics are most effective in improving endothelial function? Following a screening process, the authors meta-analyzed 51 chronic exercise studies. The three types of exercise modalities significantly improved endothelial function; however, the highest effect on endothelial function was for aerobic (ES = 2.79, CI95% = 2.12, 3.45), followed by resistance (ES = 2.52, CI95% = 1.10, 3.93), and concurrent (ES = 2.07, CI95% = 0.70, 3.44) exercises. In addition, heart disease patients improved endothelial function using any of the exercise modalities; however, healthy participants remarkably enhanced endothelial function after performing resistance exercise (ES = 1.87, CI95% = 0.18, 3.56) as opposed to aerobic (ES = 1.57, CI95% = -1.95, 5.09) and concurrent (ES = -0.90, CI95% = -2.41, 0.60) exercises.The chronic effect of different exercise modalities on endothelial function and arterial diameter has been summarized; however, the immediate or acute effect has not been extensively reported. Therefore, the purpose of this meta-analysis was to investigate the acute effect of different exercise modalities on arterial endothelial diameter.
2. Materials and Methods
2.1. Design
- The present aggregate-data meta-analysis followed procedures suggested by Borenstein, Hedges, Higgins and Rothstein [16] and Moher, Liberati, Tetzlaff and Altman [17].
2.2. Information Sources and Search Strategy
- The literature search for potential studies was performed from August 2015 to February 2016. The electronic databases searched were PubMed, Springer Link, Science Direct, SAGE Journals, and EBSCOhost (including several other databases such as Medline, Sport Discuss, and Academic Search Complete). The reference list from selected articles was reviewed for potential eligible studies. The following keywords were used for searching the databases: “acute exercise”, “FMD”, “flow-mediated dilation”, “endothelial function”, “exercise”, “vascular”, and the combination of these.
2.3. Eligibility Criteria and Study Selection
- Studies were meta-analyzed if they met the following inclusion criteria: a) experimental studies, b) acute intervention, c) studies involving human healthy adults, d) articles published in English language, e) studies reporting at least pre- and post-FMD mean measurements, standard deviation, and sample size, and f) articles published in peer-reviewed journals. Studies were excluded if they were: a) chronic effect studies, b) studies performed in children and adolescents, c) animal studies, d) diseased populations (e.g., hypertension, heart disease, and chronic disease). The authors selected and retrieved the information from the individual articles. Disagreements as to whether include or exclude an article was solved by consensus.
2.4. Data Collection Process
- The following information was extracted from the selected studies and coded into an Excel® spreadsheet: a) study design, b) study quality in a scale from 0 to 5 (i.e., randomization, correct allocation of randomization, control group, pre- and post- measurements, description of experimental death, sample size), c) participant’s characteristics (i.e., age, gender, fitness level, smoking status), d) characteristics of the treatment (i.e., exercise type and modality, duration, repetitions, series and rest), and e) measurements (i.e., at least pre- and post-measurements).
2.5. Risk of bias
- The risk of bias was assessed based on a list of items such as eligibility criteria specified, randomization specified, groups similar at baseline, report of adverse events, exercise attendance reported, and between-group statistical comparisons reported for the main outcome variable (i.e., FMD) [18].
2.6. Summary Measures
- The primary outcome was FMD, and a within-group ES was computed from a standardized mean difference according to Borenstein, et al. [16].
2.7. Heterogeneity and Small-study Effects
- Cochran’s Q test and the I2 statistic were used to assess heterogeneity and inconsistency across included studies [16]. The Q statistic was tested at p < 0.10 and the I2 values were considered very low (< 25%), low (25 to < 50%), moderate (50 to < 75%), and large (> 75%). The influence of small-study effects was determined by visual inspection of a funnel plot and Egger’s regression [19, 20].
2.8. Moderator Variables
- Age, gender, fitness level, body mass index (BMI = weight in kg/height in m2), exercise intensity and mode, as well as duration were coded as potential moderator variables. All possible ES’s were coded when a study reported data on several time points (i.e., repeated measures).
2.9. Statistical Analysis
- Statistical analysis was carried out using the IBM-SPSS software, version 21 (IBM Corporation, Armonk, New York), and MetaXL software, version 5.3 [21]. Descriptive statistics are presented as mean and standard deviation (M ± SD), unless otherwise noted. For inferential analysis, statistical significance was set a priori at p < 0.05. The CI95% were computed to determine whether there is statistically significant difference on the ES between groups (i.e., control vs. experimental). Effect sizes were evaluated as suggested by Cohen [13].
3. Results
- A flow diagram depicting the search strategy process and the final number of selected studies (n = 13) is presented in figure 1. The total number of participants analyzed was 378 (245 males, 133 females) with a mean number of participants per study of 29 (range = 10 to 74).
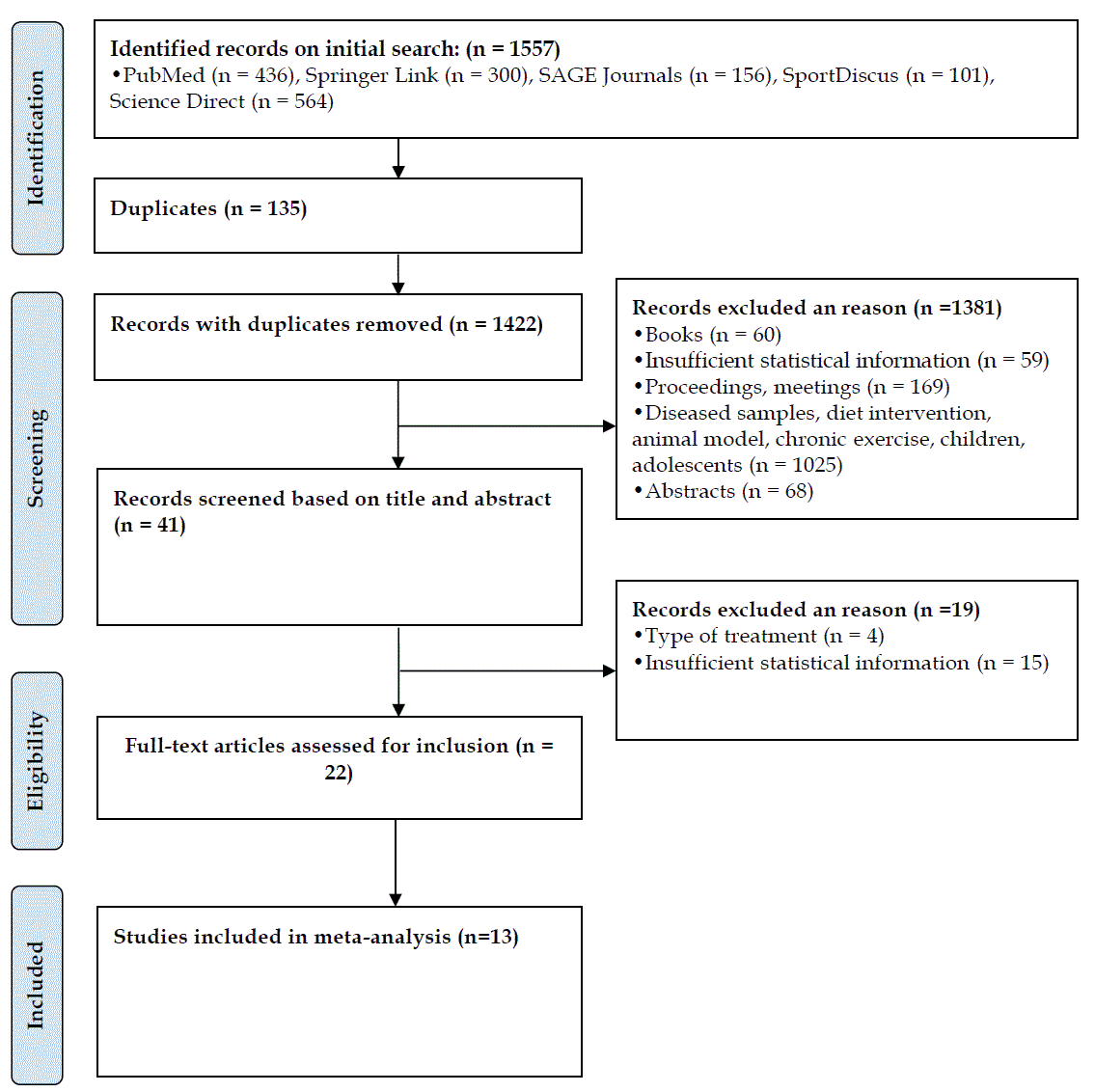 | Figure 1. Study search and selection flowchart [17] |
3.1. Characteristics of Included Studies
- The main characteristics of the studies included in the analysis are shown in table 1. The mean age of the population was 33.6 ± 2.7 yr. (range = 18 to 64 yr.). Data were extracted from 13 studies; in six studies participated males, in six studies a mixed sample and one study included only females. Twelve studies showed very poor and poor quality and only one study showed good quality.
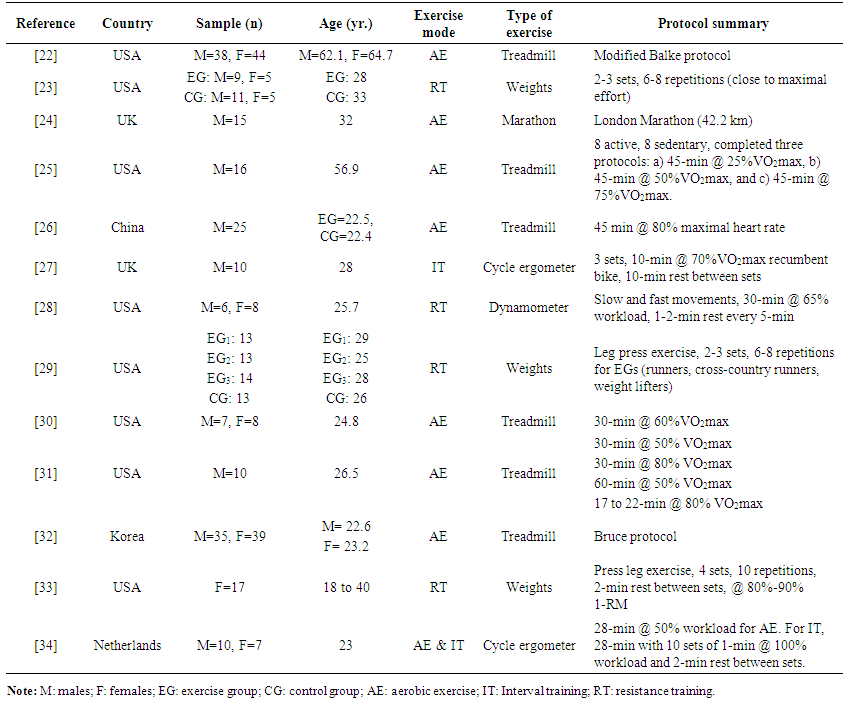 | Table 1. Characteristics of the exercise studies included in the meta-analysis (n = 13) |
3.2. Meta-analysis of Aerobic Training Studies
- Twenty-seven ES’s were computed for a total of 254 participants. There was no significant effect of aerobic exercise interventions on arterial diameter (ES = 0.27, CI95% = -0.08, 0.62, p = 0.11, Figure 2). In general, studies provided homogenous results (Q = 14.19, p = 0.97, I2 = 0%). Moderator variable analysis was not performed since the ES was non-significant.
 | Figure 2. Forest plot of the effect of aerobic exercise on arterial diameter. The diamond indicates the overall ES |
3.3. Meta-analysis of Resistance Training Studies
- Nine ES’s were computed for 114 participants. There was no significant effect of resistance training exercise interventions on arterial diameter (ES = -0.15, CI95% = -0.76, 0.46, p = 0.25, Figure 3). In general, studies provided homogenous results (Q = 0.39, p = 1.00, I2 = 0%). Moderator variable analysis was not performed since the ES was non-significant.
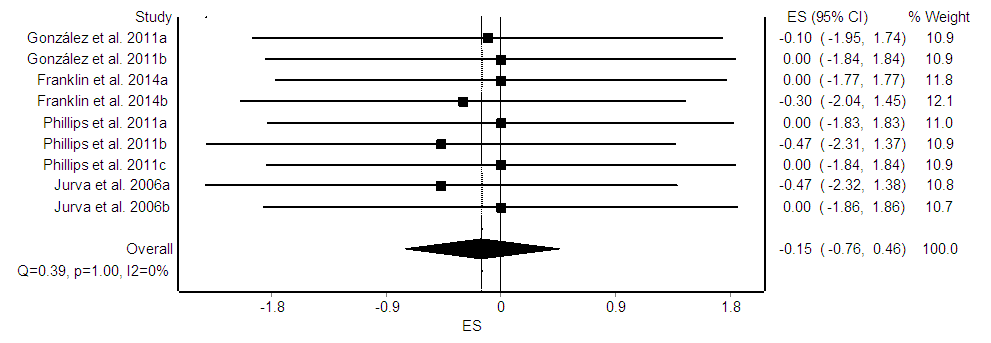 | Figure 3. Forest plot of the effect of resistance training exercise on arterial diameter. The diamond indicates the overall ES |
3.4. Meta-analysis of Interval Training Studies
- Three ES’s were computed for 35 participants. There was no significant effect of interval exercise interventions on arterial diameter (ES = 0.23, CI95% = -0.82, 1.27, p = 0.15, Figure 4). In general, studies provided homogenous results (Q = 0.01, p = 0.99, I2 = 0%). Moderator variable analysis was not performed since the ES was non-significant and the small number of available studies.
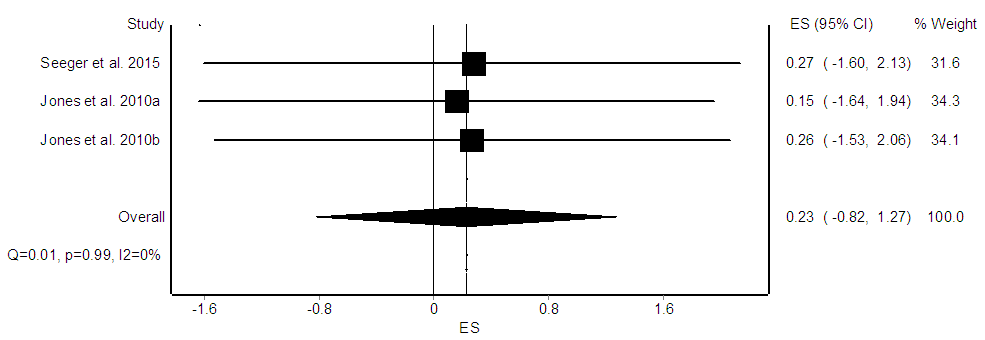 | Figure 4. Forest plot of the effect of interval exercise on arterial diameter. The diamond indicates the overall ES |
4. Discussion
- The purpose of this aggregate meta-analysis was to determine the effect of acute aerobic, resistance and interval exercise modes on arterial diameter as measured by the FMD technique. The main findings based on Cohen’s classification of ES’s were non-significant small effects of aerobic and resistance training exercise and trivial effects of interval exercise on arterial diameter [13].The findings on the effects on endothelial function in the aerobic and resistance exercise modalities agree with others [35]. Resistance exercise is known for acutely increasing blood pressure [36]; which contributes to acute changes in arterial diameter as observed on patients with peripheral artery disease [37]. However, changes in arterial diameter might be influenced by participant’s training or fitness status. For instance, endothelium-dependent vasodilation has been found to be reduced following acute exercise in sedentary but not in exercising adults [38]. In this study, the influence of moderator variables was not performed given the lack statistical significance of ES’s of aerobic and resistance training exercises and the limited number of available studies on interval training interventions.Previous meta-analytic reviews [14, 15], reported significant global ES’s for the effects of aerobic and resistance training exercises on arterial diameter, but not for the interval exercise; however, these meta-analytic reviews were focused on the chronic effects of training. In addition, these reports studied a different population; Black, et al. [15], meta-analyzed only male athletes, whereas [14], studied men and women, healthy and diseased. Yet, the analysis of moderator variables did not show a significant global ES for the category of health status. The acute effect of exercise was not included in that study.Several methodological issues must be addressed to further explore the possibility of completing a new systematic review with or without meta-analysis. Greyling, van Mil, Zock, Green, Ghiadoni and Thijssen [39], conducted a systematic review to determine the association between adherence to current guidelines for FMD measurement and its reliability. With 27 included studies and a total of 1537 subjects, it was found that the adequate use of the current guidelines reduced the measurement error and its variability (R2 = 0.36, p < 0.01). Since several studies analyzed in the present meta-analysis did not report a detailed description of the participant preparation, image acquisition and analysis, and laboratory test coefficient of variation, among others, it is unlikely that valid, reliable and grounded conclusions regarding the acute effects of exercise on vascular response can be reached at this time.The ES’s found in the present study were trivial, small and lacked of statistical significance. Even if ES’s would have been significant, the fail-safe for aerobic (n = 182), resistance (n = 34), and interval (n = 17) suggest a weak strength of data reported so far given the elevated number of studies being carried using those exercise paradigms. We might reasonably expect increases in statistical power and perhaps clinical significance if more methodologically sound studies are published in a near future. At this time, the poor quality of the published studies on the acute effects of exercise on arterial endothelial diameter prevents from reaching strong conclusions. Authors are encouraged to follow proper guidelines not only for measuring flow-mediated variables, but also for reporting details on the exercise interventions. For instance, some of the information still required to meta-analytic summarize findings on this topic include the number of sets, repetitions, rest between sets, and workload estimations. The lack of these data prevented us and will prevent others from studying moderator variables.
5. Conclusions
- In conclusion, acute aerobic, resistance and interval exercise training do not affect arterial endothelial diameter. Methodologically sound studies are still needed to further explore the potential effects of a single exercise session on vascular health. Comprehensive details on the exercise prescription required to replicate and later summarize studies is needed.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML