-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2018; 8(9): 226-229
doi:10.5923/j.ajmms.20180809.02

Intensive Therapy of Renal Transplant Rejection-Associated Pulmonary Infections
Ibadov R.A., Ibragimov S.Kh., Matkarimov Z.T., Bakhritdinov F.Sh.
National Specialized Scientific-Practical Centre for Surgery, Named after Academician V. Vakhidov, Tashkent, Uzbekistan
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Research objective: to analyze intensive therapy results of pulmonary infectious complications after renal transplant. Materials and methods: Examination and treatment results of 96 patients after heterotopic renal transplantation between 2010-2018 have been retrospectively studied. Pulmonary complications with the development of bilateral interstitial pneumonia associated with renal transplant rejection was observed in 9 patients in the early and distant postoperative period. The biomaterials taken from the patients with infectious complications due to acute and chronic renal transplant rejection were studied. Results: 8 patients had a positive dynamics due to the conducted respiratory and intensive therapy. The tendency of causative agents changes and the growth of gram-negative bacteria volume has been observed for the period of study. Analysis of the isolated microorganism sensitivity revealed an increase of dominating causative agents resistance. Augmentation of leading pathogen antibiotic-resistance causes the necessity of studying antibioticograms of all strains taken from the patients to choose an adequate and effective antibiotic therapy.
Keywords: Renal transplant rejection, Immunosuppression, Pulmonary infection, Noninvasive respiratory support, Intensive therapy
Cite this paper: Ibadov R.A., Ibragimov S.Kh., Matkarimov Z.T., Bakhritdinov F.Sh., Intensive Therapy of Renal Transplant Rejection-Associated Pulmonary Infections, American Journal of Medicine and Medical Sciences, Vol. 8 No. 9, 2018, pp. 226-229. doi: 10.5923/j.ajmms.20180809.02.
1. Introduction
- The success of renal transplant depends on reaching a compromise between effective immunosuppression aimed to the prevention of a rejection crisis and preservation of immune protection of the body [1]. Rehabilitation of patients with renal transplant (RT) is a difficult clinical task and is quite often associated with several problems, one of them are infectious complications. Among these complications, pulmonary infections make up from 5% to 37% and play the important role in the structure of morbidity in recipients of allogenic kidney graft [2-4, 7]. In this view, RT patients should be included in the group with a high risk of fatal pulmonary infections [4, 8]. Irreversible lungs injuries due to excessive immunopathologic reactions to CMV-antigens, expression of specific cytotoxic lymphocytes on the infected cells of the lungs lead to destruction of the alveoli [16].When managing RT patients with pulmonary infectious complications the choice of optimum respiratory support tactics and pathogenetic therapy, in our opinion, is one of the challenges at the development of acute respiratory distress syndrome (ARDS). According to the resolution of the Congress of the European Respiratory Society (ERS 2013), application of NiCPAP (Noninvasive Constant Positive Airway Pressure) mode of artificial lungs ventilation (AVL) and intrapulmonary percussion ventilation (IpPV) is able to save patients life requiring respiratory support and considerably contributes to satisfactory intensive care outcomes in ARDS development [5, 6].The objective of this research was to define peculiar features of noninvasive respiratory and intensive therapy for patients with pulmonary infectious complications associated with acute and chronic rejection of a renal transplant.
2. Material and Methods
- The research was based on observation and treatment results of 96 patients after heterotopic renal transplantation performed between 2010 and 2018. The total number of renal transplantations was 140 and we investigated 96 of them. Bilateral interstitial pneumonia was diagnosed in 3 patients in the immediate postoperative period; in 6 patients it was developed later. The follow-up period has lasted from 1 month to 4 years.Among them 4 patients being operated in India and Pakistan were admitted to our Surgery Centre with clinical presentation of acute lung injury syndrome: in one case it was associated with acute transplant rejection, while in three other cases - due to chronic renal transplant rejection, pyelonephritis, and bacterial pneumonia followed by sepsis. The diagnosis criteria were based on regulating documents of the international interdisciplinary classification of idiopathic interstitial pneumonia developed and issued in 2013 by the American Thoracic Society and European Respiratory Society [6]. The traditional methods of isolation and identification of microorganisms, evaluation of their sensitivity to antimicrobial drugs and the dynamic control of C-reactive protein were used. Species specificity of the identified microorganisms was assessed by the standard methods using the identification sera (manufactured by "HiMedia", India). Besides, antibodies against CMV in blood serum and CMV DNA were evaluated by the quantitative PCR.The research was conducted on the following materials: urine (236 specimens), blood (195 tests), drained fluid (220 specimens), sputum (217 specimens), bronchoalveolar lavage fluid (56 specimens) and lavage fluid from the trachea (220 specimens). The efficiency of cephalosporins, aminoglycosides, fluoroquinolones, tetracyclines, carbapenems, glycopeptides, and inhibitor protected antibiotics was investigated. The obtained results were analyzed by measuring diameters of test cultures growth inhibition areas around sockets. When the inhibition area was ≤10 mm the cultures were considered resistant; when the areas were 11-14 mm - moderately resistant; the areas of 15 mm and above were interpreted as sensitive ones. Besides, the presence of antibodies against CMV in blood serum and CMV DNA were determined by PCR that showed 3.5 x 106 IU/ml. The dynamic control of C-reactive protein level was also conducted. The 3-component immunosuppressive therapy for supporting immunosuppression was initiated when per oral drug intake became possible 6-12 hours after the surgery. The recipients received the average dosages of Prograf (Tacrolimus) 1.2-1.3 mg/kg of bodyweight a day and Cell-cept (Mycophenolat Mofetil) in the dose of 2 g/a day. Low doses of corticosteroid hormones (< 0.5 mg/kg of body weight) were prescribed as the third component of the therapy. All the patients were performed antiviral, antibacterial, infusion and symptomatic therapies. The respiratory therapy was consisted of noninvasive artificial lung ventilation (ALV) according to the ATS/ERS protocol (NiCPAP ASB, PEEP 8, Trigger 3 l/min, FiO2 50%) with breaks during which PEEP mask and IPV in the mode of low and moderate percussion + nebuliser therapy were used. The VELA ventilator (VIASYS HEALTHCARE, INC.) was used to conduct NiCPAP. Mechanical percussive ventilation of the lungs was performed by IPV-HCBI-PHASIC IMPULSATOR (Percussionaire).
3. Results
- The analysis of the controlled respiratory indices dynamics on the 3rd, 5th and 8th days showed a significantly rapid increase in the levels of SaO2, paO2 and paO2/FiO2 versus the reference values (Fig. 1).
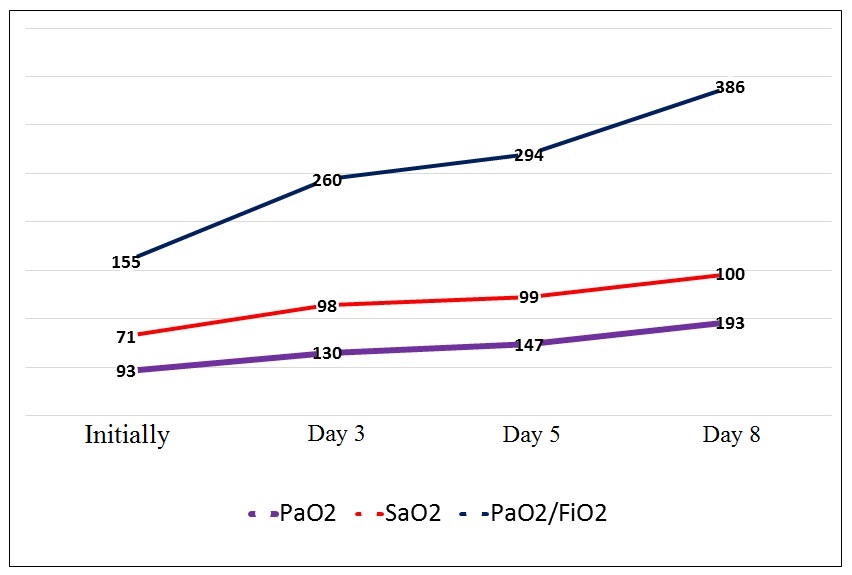 | Figure 1. The respiratory indices dynamics |
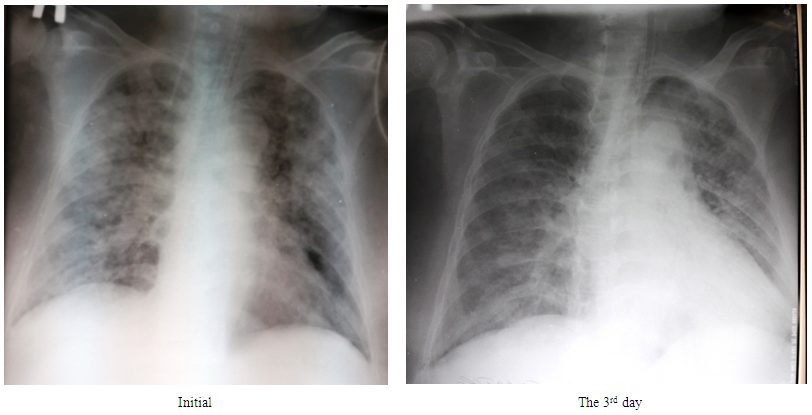 | Figure 2. The X-ray pattern dynamics in 36-years old patient with bilateral interstitial pneumonia, moderate ARDS and chronic rejection of renal transplant taken from a live related donor |
4. Discussion
- The above presented data show the leading position of respiratory diseases in the structure of severe infections in patients with renal transplant rejection. Growing resistance of infectious causative agents requires the constant monitoring of microflora composition and sensitivity. The 1st month after transplantation is associated predominantly by nosocomial bacterial infections [9]. As for nosocomial infections in the ICU, in our research, Acinetobacter spp. dominates among the causative agents having a high resistance to all groups of antibacterial drugs. For instance, for the last decade it has been identified in 5.4-39%.The second post-transplant period (from 1 to 6 months) is characterized by the dominating of opportunistic pathogens as a result of the maximum sustained immunosuppression. In the late post-transplant period the transplant function is usually stable in spite of immunosuppression level reduction. Herewith, the risk of viral and opportunistic infections is high only in patients with chronic rejection or recurrent episodes of acute rejection [9]. According to our study results, there was a significant frequency growth of Candida genus fungi occurence (7-34%).ALV, the presence of arteriovenous fistulas, diabetes mellitus, preoperative dialysis therapy and post-transplantation immunosuppression can be considered as risk factors of pulmonary complications development in the patients with renal transplants [10-15].Against the background of patients immune status abnormalities and a progress of the respiratory status at the standard approach to treatment trachea intubation and application of the traditional prolonged ALV with a high risk of ventilator-associated pneumonia development was predicted. And that is why, the entire complex of noninvasive respiratory therapy was successfully used. The application of NiCPAP and prlonged ALV was based on the following:- noninvasive ALV with continuous positive end-expiratory pressure;- mobilization of the respiratory ways obstructed by secretion products, edema of the mucous and sub-mucous membranes, and a spasm of the bronchial tubes; - a creation of bilateral adequate alveolar ventilation to improve oxygen supply and carbon dioxide removal; - mechanical intermixture of the intrapulmonary gases with the use of diffuse intrapulmonary percussion for improving endotracheal diffusion of oxygen and mobilization of carbon dioxide in the lungs’ peripheral areas; - providing essential periodic “convectional respiration flow” to remove СО2.
5. Conclusions
- The identification of risk factors of bacterial and fungal infectious complications development after renal transplantation allowed to carry out targeted preventive actions both before and after renal transplant and it led to a decrease of such complications frequency.The application of and prolonged ALV in combination with hemodialysis is an alternative treatment tactics in the patients with bilateral interstitial pneumonia and interstitial hypostasis associated with rejection of renal transplant. The range of ventilating technology in patients with a pulmonary pathology allows to optimize therapy, to protect respiratory ways, to avoid negative consequences of ALV.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML