-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2018; 8(9): 219-225
doi:10.5923/j.ajmms.20180809.01

Role of Acute-phase Proteins (Orsomucoid 2 and hs-CRP) in Lean Type 2 Diabetic Patients with and without Subclinical Atherosclerosis
Mona Abd EL Rahman Eldosoky 1, Amany M. Abdallah 2, Noha Abdel-Rahman Eldesoky 3, Maha A. Hassan 2, Gihan A. Eldesoky 4
1Clinical Pathology Department, Faculty of Medicine for Girls, Al-Azhar University, Egypt
2Internal Medicine Department, Faculty of Medicine for Girls, Al-Azhar University, Egypt
3Biochemistry Department, Faculty of Pharmacy for Girls, Al-Azhar University, Egypt
4Anesthesia and Intensive Care Department, Faculty of Medicine for Girls, Al-Azhar University, Egypt
Correspondence to: Noha Abdel-Rahman Eldesoky , Biochemistry Department, Faculty of Pharmacy for Girls, Al-Azhar University, Egypt.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

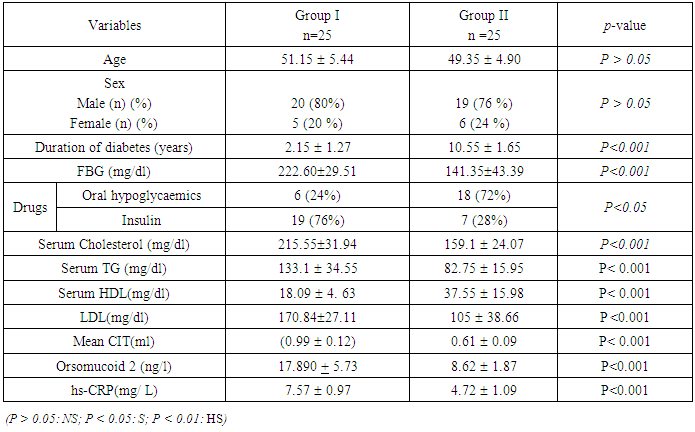
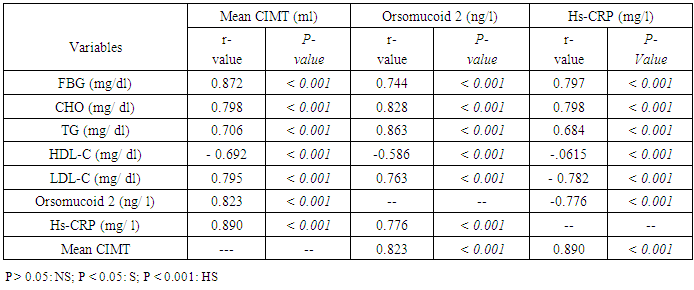
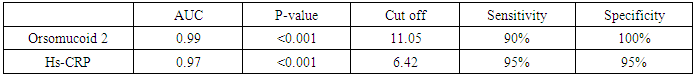
Background: Type 2 Diabetes Mellitus (T2DM), is a global public health crisis that threatens the economies of all nations, particularly developing countries. Cardiovascular complications are the main cause of mortality in T2DM patients. Objective: The aim of this study is to explore the role of acute-phase proteins (Orsomucoid 2 and hs-CRP) as predictors for subclinical atherosclerosis and their relation with dyslipidaemia and Carotid intima media thicknes (CIMT) in lean T2DM patients. Methods: 50 diabetic normal weight patients (22 males and 28 females) were recruited from internal medicine department, AL-Zahraa hospital and divided into two groups: Group 1; twenty five type 2 diabetics with increased CIMT (≥ 0.9 mm) and Group 2; twenty five diabetic patients with normal CIMT (<0.9 mm). All the participants were subjected to full medical history, full clinical examination, and routine Laboratory investigations: Serum fasting glucose, urea, creatinine, uric acid, potassium, calcium, phosphorous, bilirubin, ALT, AST, albumin, complete lipid profile, hs-CRP and Orsomucoid 2 (ORM2) assay. Electrocardiogram (ECG), Echocardiography and B-mode ultrasonography of left and right carotid arteries were also assessed. Findings: Serum orsomucoid 2 and hs-crp were significantly higher in group 1 compared to group 2 at (p≤0.001). Serum orosomucoid 2 at cut off 11.05 ng/L could discriminate patients with and without subclinical atherosclerosis. There was Positive correlation between serum orsomucoid level, hs-CRP and each of CIMT, LDL-C, and TG while negative correlation was found with HDL-C. Mean CIMT presented positive correlation with total cholesterol level, triglycerides and low-density lipoprotein. Conclusions: Our findings suggest that orsomucoid 2 and hs-CRP could be used effectively as biomarkers for subclinical atherosclerosis in lean T2DM patients.
Keywords: Carotid intima media thickness, Diabetes mellitus, Atherosclerosis, Orsomucoid, hs-CRP
Cite this paper: Mona Abd EL Rahman Eldosoky , Amany M. Abdallah , Noha Abdel-Rahman Eldesoky , Maha A. Hassan , Gihan A. Eldesoky , Role of Acute-phase Proteins (Orsomucoid 2 and hs-CRP) in Lean Type 2 Diabetic Patients with and without Subclinical Atherosclerosis, American Journal of Medicine and Medical Sciences, Vol. 8 No. 9, 2018, pp. 219-225. doi: 10.5923/j.ajmms.20180809.01.
Article Outline
1. Introduction
- Atherosclerotic cardiovascular disease (CVD) is a modifiable complication of altered glucose metabolism that leads to considerable disease burden, increased health services use and premature morbidity and mortality [1]. Di Carli and Hachamovitch [2] reported that 20–50% of patients with diabetes have silent CHD (coronary heart disease). Once CHD is symptomatic in diabetes, morbidity and mortality are high and significantly worse than in patients without diabetes. Therefore, precise detection of subclinical atherosclerosis in diabetic patients could allow effective therapeutic intervention to slow or reverse the progression of atherosclerotic disease [3]. Carotid intima-media thickness (CIMT) is a noninvasive index of subclinical atherosclerosis [4]. Increased CIMT correlates with cardiovascular outcomes such as increased risk of stroke and coronary artery diseases [5]. Factors such as age and high levels of low-density lipoprotein cholesterol (LDL-C) are associated with increased CIMT in diabetic patients [6]. An important potential link between diabetes and atherosclerosis may involve inflammatory factors. Markers of inflammation are considered surrogates of asymptomatic atherosclerosis, as inflammation has a crucial role in the atherogenic process. Several inflammatory markers may be associated with diabetes, including C-reactive protein (CRP) and orosomucoid (ORM) [7]. CRP induces endothelial cell adhesion molecules and increases endothelial cell monocyte chemoattractant protein-1, inhibit nitric oxide synthesis, increases plasminogen activator inhibitor (PAI)-1 expression, and leads to endothelial dysfunction [8]. As inflammation proceeds, the risk for vascular complications increases. ORM, also known as alpha 1 acid glycoprotein, is a family of glycoproteins that includes two main members: ORM1 and ORM2. ORM2 is an acute-phase protein that is mainly biosynthesized and secreted by liver. ORM2 is considered an anti-inflammatory and immunomodulatory factor due to its anti-neutrophil and anti-complement activity [7]. The anti-inflammatory, immunomodulating, and angiogenic effects of ORM2 are possibly important in the pathogenesis of cardiovascular disease as elevated levels of ORM have been associated with coronary disease and stroke [9].Cardiovascular risk in normal-weight diabetic patients may be under assessed for years because of their normal BMI. The major pathophysiology in this group appears to be rapid beta cell failure as opposed to insulin resistance [10]. Our study investigated whether serum orsomucoid 2 and hs-CRP were related to individual cardiovascular disease risk in normal-weight (lean) T2DM patients without symptomatic cardiovascular disease. We hypothesized that although the patients were normal-weight, markers of inflammation would be significantly related to cardiovascular risk independent of body composition.
2. Materials and Methods
2.1. Study Population
- This cross-sectional study included fifty normal weights T2DM patients (BMI 18- 24.9 kg/m2) recruited from internal medicine department of AL-Zahraa hospital, AL-Azhar University, Cairo, Egypt during the period from (August 2017 to February 2018). Written informed consent was obtained from all subjects. All the participants were subjected to the following: (1) Full medical history (2) Full clinical examination and BMI calculation using the formula “observed weight divided by height squared “(kg/m²). Height was recorded to the nearest 0.1 cm and Body mass was obtained using a digital scale to the nearest 0.1 kg. Normal weight (lean) was defined as BMI 18.5 to 24.9 kg/m2 [12], (3) Routine Laboratory investigations: Serum fasting glucose, urea, creatinine, uric acid, potassium, calcium, phosphorous, bilirubin, ALT, AST and albumin to exclude any renal or hepatic abnormality, triglyceride (TG), total cholesterol, low density lipoprotein (LDL), high density lipoprotein (HDL) using fully automated chemistry analyzer (Cobas integra 400 plus. Roche Diagnostics, Germany). (4) hs-CRP and ORM2 assay measured by ELISA according to the manufacturer’s instructions. (5) Electrocardiogram (ECG), Echocardiography and B-mode ultrasonography of left and right carotid arteries to determine mean CIMT. Patients were classified into 2 groups: 25 T2D patients with increased mean CIMT (mean CIT ≥ 0.9 mm) and 25 T2D patients with normal CIMT (mean CIT< 0.9 mm). Diabetes was diagnosed according to the American Diabetes Association criteria [11]. Exclusion criteria were the presence of any signs or history of clinical cardiovascular disease such as angina, myocardial infarction, valvular diseases, ventricular arrhythmias, and heart failure. Patients with any clinical evidence of infection, history of allergies, rheumatoid arthritis, recent trauma, surgery, liver dysfunction, connective tissue disease, or other autoimmune disorders that could influence the studied parameters were excluded from the study. Underweight diabetic patients and patients with low body weight as a consequence of wasting diseases such as malignancies, tuberculosis, alcoholism or chronic pancreatitis were also excluded. All procedures performed in the study involving human participants were in accordance with the ethical standards of the institutional and national research committee (ERC number 3) and with the 1964 Helsinki declaration and its later amendments (version 2013 for medical researchers involving human subjects).
2.2. Sample Collection and Routine Lab Analysis
- Eight ml of venous blood were drawn after fasting nine hours, centrifuged to separate serum, the serum obtained was divided into 2 portions; the 1st portion used for the measurement of Lipid profile [total cholesterol, triglyceride, high density lipoprotein, Low density lipoprotein], fasting glucose, urea, creatinine, uric acid, potassium, calcium, phosphorous, bilirubin, ALT, AST and albumin using chemistry autoanalyzer (Cobas integra 400 plus, Roche diagnostics, Germany), the 2nd portion was stored at −20°C until hs-CRP and orsomucoid 2 time of assay using enzyme-linked immunosorbent assay.
2.3. Measurement of CIMT
- CIMT was measured by B-mode ultrasound using a linear transducer (7.5–10 MHz) by a single sonographer. CIMT is presented as a mean value of two measurements from both sides of the common carotid arteries. Elevated CIMT as measured by ultrasound is defined as ≥ 0.9 mm for common carotid artery in middle-aged adults [13].
2.4. High Sensitive-CRP and Orsomucoid Assay
- Enzyme-Linked Immunosorbent Assay (Sandwich ELISA) was used to detect and quantify levels of orsomucoid 2 and hs-CRP. A polyclonal antibody specific for orsomucoid or hs-CRP has been pre-coated into the microwells. The orsomucoid or hs-CRP protein in samples was captured by the antibody coat after incubation. Following extensive washing, a specific monoclonal antibody was added to detect the captured orsomucoid or hs-CRP. For signal development, horseradish peroxidase (HRP)-conjugated antibody was added, followed by Tetramethyl-benzidine (TMB) reagent. Solution containing sulfuric acid was used to stop color development and the color intensity which is proportional to the quantity of bound protein was measured at 450 nm. The detection range of hs-CRP was 0.068-8.2 mg/L while that for orsomucoid 2 was 3.7 – 120 ng / L. The kits for hs-CRP were provided by DRG International Inc (841 Mountain Avenue, Springfield, New Jersey, USA) while that for orsomucoid 2 was supplied by Kono Biotech C., Ltd (Shijia North Road, Zhejiang, China).
2.5. Statistical Methods
- Data were coded and entered using the statistical package SPSS version 25. Data was summarized using mean and standard deviation for quantitative variables and frequencies (number of cases) and relative frequencies (percentages) for categorical variables. Comparisons between groups were done using unpaired t test. For comparing categorical data, Chi square test was performed. Exact test was used instead when the expected frequency was less than 5. Correlations between quantitative variables were done using Pearson correlation coefficient. ROC curve was constructed with area under curve analysis performed to detect the best cutoff value of orsomucoid 2 or hs-CRP for detection of subclinical atherosclerosis. P-values less than 0.05 were considered statistically significant.
3. Results
- This study was conducted on 50 lean T2DM patients, categorized into 2 groups; group (1): T2DM patients with high CIMT (≥ 0.9 mm) (n=25), group (2): T2DM patients with normal CIMT (<0.9 mm) (n=25). All patients had normal body mass index (18- 24.9). The Demographic data, clinical characters and biochemical parameters of all subjects are presented in Table 1.
|
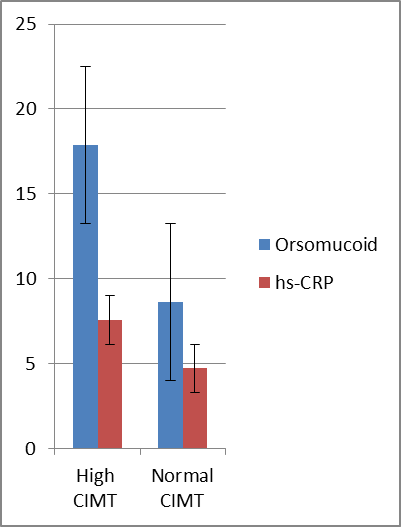 | Figure 1. Orsomucoid 2 and hs-CRP were significantly higher in diabetics with high CIMT |
|
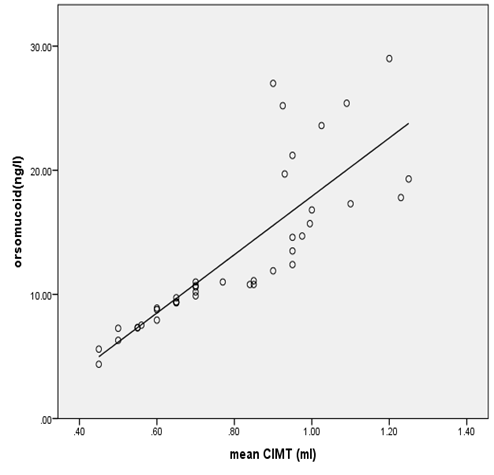 | Figure 2. Correlation between orsomucoid 2 level and mean CIMT in all diabetic patients |
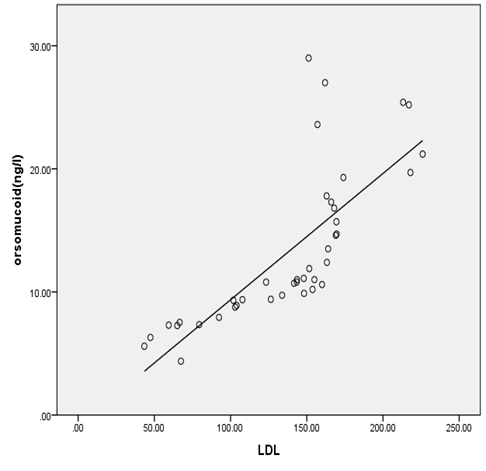 | Figure 3. Correlation between Orsomucoid 2 and LDL cholesterol in all diabetic patients |
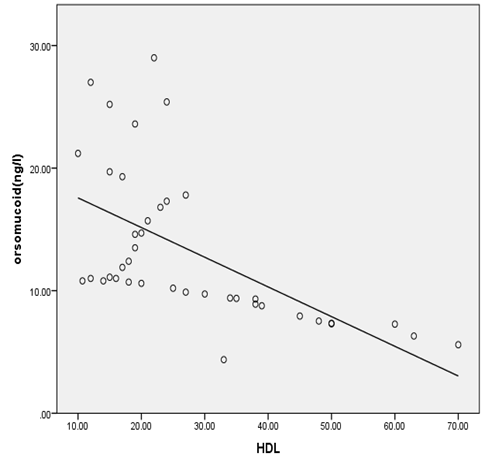 | Figure 4. Correlation between orsomucoid 2 and HDL cholesterol in all studied diabetic patients |
|
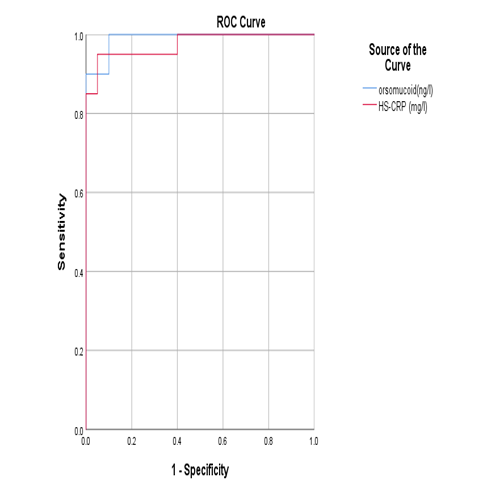 | Figure 5. ROC curve showing the diagnostic performance of orsomucoid 2 and hs-CRP for discriminating diabetic patients with subclinical atherosclerosis |
4. Discussion
- The prevalence of diabetes mellitus has increased globally over the past decade [10]. T2DM is associated with marked increase in the risk of atherosclerotic diseases including coronary heart disease, peripheral arterial disease, and cerebrovascular disease. Although atherosclerosis is usually detected when patients present with a major cardiovascular event, identification of atherosclerosis when it is still in its subclinical stages allows early intervention to prevent subsequent cardiovascular events [14]. Most patients with T2DM are obese or overweight. Few researches studied T2DM with a normal BMI. Because these individuals are normal-weight, they may not be aware of cardiovascular risk and are not usually expected to have CVD complication.B-mode ultrasonography measurement of the CIMT is considered a validated measurement of cardiovascular risk and atherosclerotic disease burden [15]. Inflammation has an important role in the development and progression of atherosclerosis. In the course of this process, many acute-phase proteins are released expressing the non-specific reaction of the body to tissue injury [16]. Orosomucoid 2 is an acute-phase serum protein produced by the liver in response to infection and inflammation. The anti-inflammatory, immunomodulating, and angiogenic effects of ORM 2 could be important for cardiovascular disease pathogenesis [9]. In this study, we aimed to assess orsomucoid 2 level and hs-CRP and their relation with CIMT as predictors of subclinical atherosclerosis in lean T2DM patients.Our study reported a male predominance in the studied T2DM patients (78%).This was in accordance with the data recorded by Hartmann et al., Costanzo et al. and Coleman et al. [17, 18, 19] who reported high prevalence of male gender in lean T2DM. Hartmann et al [17] recorded that lean diabetics had higher frequency of insulin use indicating rapid beta cell failure. In this study, insulin use was more prevalent in the group with high CIMT (76%) while treatment with oral hypoglycaemic drugs was more in the second group (72%).This may be explained by longer disease duration of this group with progressive failure of beta cells.In the present study, there was a statistically significant difference between the two groups regarding serum ORM 2 level; being higher in high CIMT group compared with diabetic patients with normal CIMT. In accordance with our results, Berntsson et al [20] noted high concentrations of orosomucoid associated with carotid plaques in subjects without cardiovascular disease. Yin et al [21] found that orsomucoid was one of the top markers of atherosclerotic cardiovascular disease. In the present study, statistically significant higher levels of hs-CRP were noted in group (1) compared to group (2) (p < 0.001). This comes in agreement with Gao et al. [22] who reported that increased hs-CRP levels in T2DM patients point to involvement of inflammation in the pathogenesis of diabetes and early atherosclerosis. Khera et al [23] and Scillinger et al [24] stated that higher CRP levels were associated with increased prevalence of subclinical atherosclerosis and hence CRP appears to be a sensitive biomarker for quantification of cardiovascular risk.In the present study, significant positive correlations were noted between CIMT and each of FBG, total cholesterol, TG, LDL-C while negative correlation was observed between CIMT and HDL-C. Similarly Gao et al [22] reported that high FBG was one of the determinants of increased CIMT and that The mean CIMT increased by 2.0 μm with each 1‐mmol/L increase in FBG level in low‐income Chinese population. Similarly, Abd El-Hafez et al [25] recorded direct correlation between CIMT and FBG. Bashir et al [26] reported positive association between CIMT and each of total cholesterol and LDL-C. They also stated that chronic hyperglycaemia has been found to be an independent risk factor for the carotid thickening. In addition, Frost et al [27] observed that total cholesterol was associated with increased CIMT in newly diagnosed T2DM. Dyslipidemia is a crucial part of the diabetic metabolic abnormalities as it accelerates the processes that results in plaque formation in the circulation. Kota et al. [28] found positive correlation between CIMT and each of total cholesterol, LDL cholesterol and triglycerides. They concluded that routine measurement of CIMT is of great value for risk stratification in diabetic patients. Paradoxical to our results, Bashir et al [26] found no relation between CIMT and TG or HDL-C. In the present work, there was significant positive correlations between each of orsomucoid 2, hs-CRP and common CIMT which may be attributed to progressive inflammation in the ongoing atherosclerotic process. Schillinger et al [24] reported that the degree of inflammation measured by specific biomarkers may reflect the disease activity and can predict progression of atherosclerosis. Therefore, Acute-phase reactants as C-reactive protein (CRP) and ORM 2 may help as indirect measures of the cytokine-dependent inflammatory process in the arterial wall. In agreement with our results, Ali and Hadidi [29] found significant positive correlation between hs- CRP and CIMT reflecting the connection between markers of inflammation and atherosclerotic cardiovascular risk. On contrary to our results, Michael et al. [30] found that increased hs-CRP in non obese individuals was mildly associated with increased CIMT. They suggested that the causal role of CRP in CVD relies on pathways other than increased subclinical atherosclerosis.In the current study, significant positive correlation between orsomucoid 2 and hs-CRP was observed. In accordance with our results, Berrahmoune et al. [31] recorded similar results and recommended their combined use for assessment of cardiovascular risk.In the present study, significant positive correlations were observed between serum orsomucoid 2, hs-CRP levels and each of FBG, triglycerides, total cholesterol, LDL-C (P < 0.001). Similarly, Faraj et al. [32] examined the association between several traditional risk factors and plasma inflammatory markers including hs-CRP and orosomucoid 2 in non-diabetic overweight and obese postmenopausal women and found them positively correlated with triglycerides, total cholesterol and negatively correlated with HDL-C.In order to evaluate the diagnostic performance of serum orsomucoid 2 and hs-CRP, ROC curves were carried out and we found the best cutoff value of serum orosomucoid 2 and hs-CRP that could discriminate between T2DM patients with and without subclinical atherosclerosis at 11.05 ng/L and 6.42 mg/ L respectively with a sensitivity of 90%, 95% and specificity of 100%, 95%, (AUC: 0.96, 0.93) respectively.
5. Conclusions
- Inflammatory markers including orsomucoid 2 and hs-CRP have obvious role in the prediction of subclinical atherosclerosis and hence atherosclerotic cardiovascular risk in non-obese T2DM patients, so we recommend their use routinely for screening of subclinical atherosclerosis in T2DM patients.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML