-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2018; 8(8): 191-193
doi:10.5923/j.ajmms.20180808.04

The Relevance of Cultivated Allofibroblasts Treatment on the Thymus Morphology in Burn Disease
B. A. Magrupov, A. A. Radjapov
Tashkent Medical Academy, Republican Research Centre of Emergency Medicine, Tashkent, Uzbekistan
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

One of the most perspective direction for the treatment in burn disease is a possibility of using up-to-date biotechnological methods. The article is devoted to the experimental study of cultivated allofibroblasts influence on the thymus morphology. A deep thermal burn (rate IIIB) was simulated in 46 white rats (females) with mass of 140-270 gr. The burn in the animals was simulated by application of heated up to 100°C metal plate to the skin under the etherization. Transplantation of cultivated allofibroblasts on the deep burn wounds in the rats leads to earlier relief of burn disease signs and more favorable course of a wound process: an early development of granulation, “crust” formation playing the role of bio-protector, stimulation of marginal epithelization in the wound. The application of CAF transplantation at the treatment of BD in the rats is followed by less turbulent reaction of the thymus on thermal injury with earlier normalization of morphologic signs. Thus, the use of cultivated allofibroblasts in burn disease leads to the normalization of immune system organs structure.
Keywords: Burn disease, Transplantation, Cultivated allofibroblasts, Thymus, Morphology
Cite this paper: B. A. Magrupov, A. A. Radjapov, The Relevance of Cultivated Allofibroblasts Treatment on the Thymus Morphology in Burn Disease, American Journal of Medicine and Medical Sciences, Vol. 8 No. 8, 2018, pp. 191-193. doi: 10.5923/j.ajmms.20180808.04.
1. Introduction
- Thermal injury can lead to the organs multiple dysfunction by discharging proinflammatory mediators and reactive oxygenic radicals [7]. Currently one of the most perspective direction for the treatment of burn disease (BD) is a possibility of using up-to-date biotechnological methods such as transplantation of cultivated cells of the pelt (fibroblasts) for closure extensive wound surfaces [3-4, 6]. This scientific direction is particularly actual in consideration of the severity of clinical course dependence from the area of burn injury: a long existence of open wound surfaces leads to the significant loss of proteins, electrolytes, water and heat; promotes the penetration of infection and to the reduce of immunity leads to patient’s starvation [1]. The basic burn injury is characterized by oxidative stress, continuous hypermetabolic, catabolic conditions and immunosuppression [8].
2. Aim
- In consideration of immune system interest in BD clinical course the goal of our research was to study the effect of cultivated allofibroblasts (CAF) transplantation on the thymus morphology in the rats with BD by experimental way.
3. Material and Methods
- A deep thermal burn (rate IIIB) was simulated in 46 white rats (females) with mass of 140-270 gr. The burn in the animals was simulated by application of heated up to 100°C metal plate to the skin under the etherization. The plate’s exposure time through the wipe was 16 seconds. The plate’s area made up 18-20% from the general surface of a rat’s skin. An injury of all skin layers achieved at the pointed exposure conditions.The animals were randomized in 2 groups: 1) the rats with deep thermal injury without treatment (28 ones, a control group); 2) the rats with deep thermal injury and transplantation of CAF (18 rats).We have used our own developed treatment method for burns and wounds in our investigations which included a production of primary culture of allogenic fibroblasts from embryonal tissues with posterior application of cellular meal on the wound surface.The primary culture of fibroblasts was produced by embryonal tissues fermentization of 14-17 days embryos of rats with posterior cultivation of fibroblasts in 199 culture environment containing 10-15% of embryonal calf serum, 2% α– glutamin, Hepes environment and antibiotics. The final product was the meal of cultivated allogenic fibroblasts in the culture environment with cells concentration up to 200000-300000 cells per 1 ml of the culture environment and with a high percentage of alive fibroblasts (up to 90% of cells).The rats of the 1st group were performed an excise of necrotic eschar 3 days after burn simulation. A received wound was managed without treatment. The rats of the 2nd group were performed CAF suspension 3 days after burn simulation and excise of necrotic eschar. The burn surface was covered by gauze tissue watered in saline with gentamycin after cells transplantation. The control of burn wounds healing was carried out visually (for the occurrence of purulent-inflammatory phenomena, the nature of granulations and epithelization terms) and by planimetric method.Slaughter of the animals was made 3, 6, 14, 17 and 24 days after BD. The received samples of thymus were fixed in 10% formaldehyde solution on the phosphate buffer (ph 7.2-7.4) with further filling of material by Lloyd and stain by hematoxylin and eosin. Histologic investigations were performed with the help of small and big magnification lens under the Leika light microscope.
4. Results
- The observations over the experimental animals showed that immediately after the simulation of deep thermal injury clinical presentation of severe BD was developed in all rats. Flaccidity, adynamy, frequent hypopnoe and heart rate (160-180 strikes per minute), polydipsia, polyuria were observed in all animals. The animals condition started to get better 3 days after BD.In the rats of the 1st group after the excise of necrotic eschar the wound healed slowly. A muscular layer with vascular capillaries was seen 3 days after necrectomy. The muscular layer with fascia covered by numerous vascular capillaries was seen 6 days after simulated BD. Fluid was heavy flowing out of the wound (plasmorrhea). The signs of granulation and eschar formation were registeres along the wound edge in some parts. A marginal granulation increased in its sizes and in some areas was covered by eschar 14 days after BD. There were registered plasmorrhea and serous-purulent discharge out of the wound. We registered incomplete reduction of the wound area due to wound edges contraction 24 days after the BD.Morphologic investigations revealed that there were degranulation cells in the thymus of the animals with BD which were located first in the organ capsule and stroma, then in its parenchyma. In the sequel we observed a gradual growth of thymus tissue with a formation of pathologic follicles in the rats with BD.The wound was healing faster in the animals of the 2nd group after the excise of necrotic eschar and CAF transplantation than in the 1st group. A bigger part of the wound was covered by granulations, superficial layers of which dried off with a formation of crust 3 days after CAF transplantation. A registered granulation growth was caused by CAF in the places of their graft.The process of the crust rejection from the wound edges due to the active marginal epithelization was observed and even layer of pink colored epithelium on the wound surface was seen 6 days after CAF transplantation. All clinical manifestations of BD had disappeared by that time. Then wound reparation coursed visually as cutaneous covering occurrence from wound edge on epithelial covering. The wound thoroughly healed 24 days after CAF transplantation.We observed changes in the thymus which were similar like in the control group at studying animals lymphoid organs 3 days after CAF transplantation (6 days after BD).Degranulation cells in the thymus which were diffused on the organ stroma were still observed 6 days after CAF transplantation unlike the control group.Thymus hyperplasia was not followed by pathologic follicles formation in the rats 14 days after CAF transplantation as opposed to the animals of the control group.Division on cortical and medullary substance of the thymus was not visualized 3 days after allofibroblasts’ application. Thymic lobules were predominately presented by lymphocytes bulk of which was located in the cortical substance while reticular cells mainly were located in the medullary zone. Diffuse situated degranulation cells were defined in the thymus capsules (Fig.1).
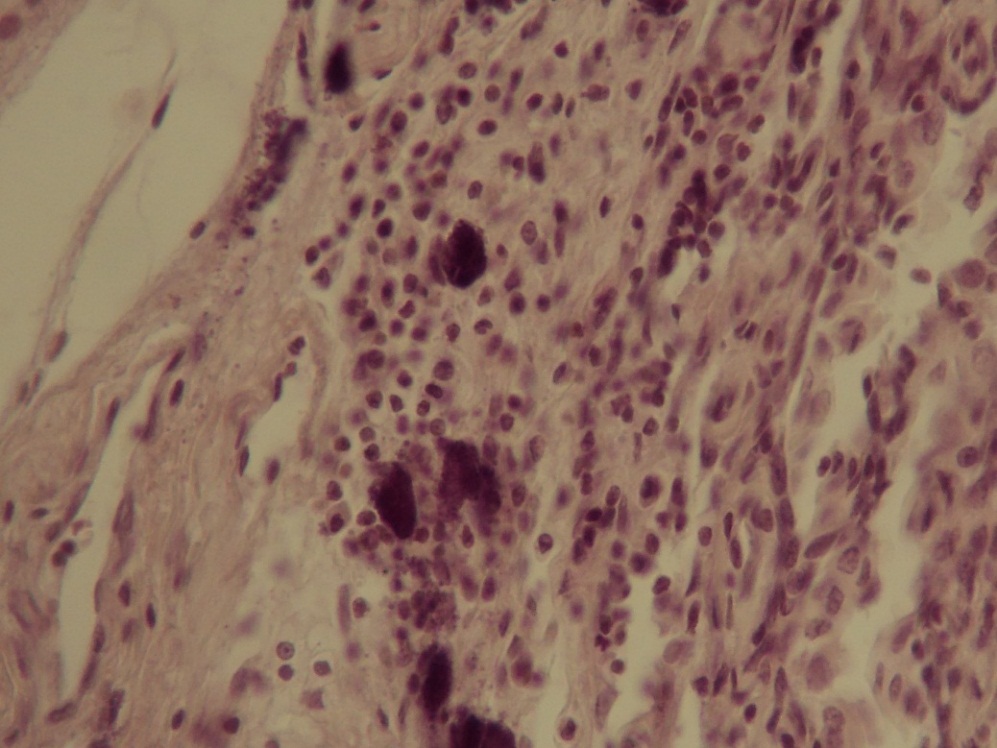 | Figure 1. Degranulation cells in the thymus (hematoxylin-eosin stain, x40) |
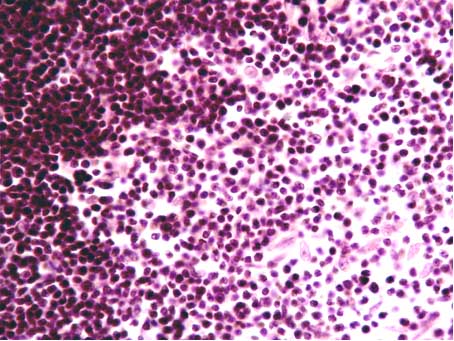 | Figure 2. Border between cortical and medullary substances of the thymus. A big quantity of reticular cells (hematoxylin-eosin stain, x40) |
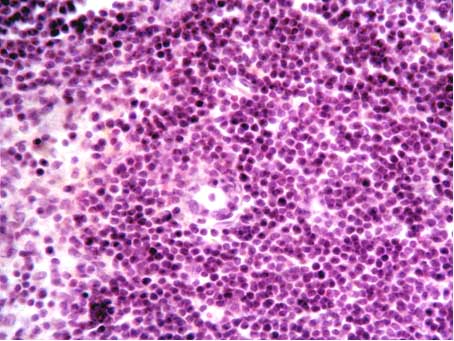 | Figure 3. Macrophages surrounded by lymphocytes in the thymus cortical substance (hematoxylin-eosin stain, x40) |
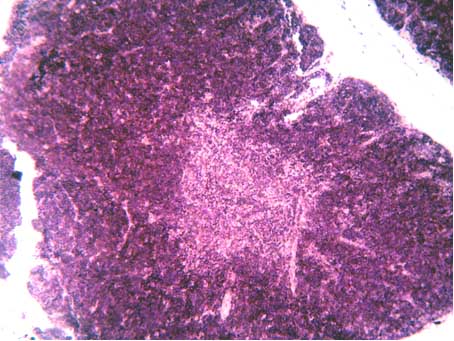 | Figure 4. Thymus lobule with well-defined border between cortical and medullary substances (hematoxylin-eosin stain, x40) |
5. Conclusions
- Burn disease in the rats is followed by evident reaction of the thymus as thymus pathologic hyperplasia lymphoid follicles formation. CAF transplantation on the deep burn wounds in the rats with BD leads to earlier relief of burn disease signs and more favorable course of traumatic period: granulations early formation, crust formation which plays the role of bio-protector, stimulation of marginal epithelization in the wound. The application of CAF transplantation at the treatment of BD in the rats is followed by less turbulent reaction of the thymus on thermal injury with earlier normalization of morphologic signs. Thus, the use of cultivated allofibroblasts at burn disease leads to the normalization of immune system organs structure.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML