-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2018; 8(8): 165-170
doi:10.5923/j.ajmms.20180808.01

Implications of Lower and Upper Respiratory Tract Infections (LRTI and URTI) in Generalized Acute Respiratory Tract Infection (ARI) among Paediatric Patients Attending National Hospital Abuja, Nigeria
Nehemiah Edem Okon , Ajobiewe Helen , Ajobiewe Joseph
Biological Sciences Department, Bingham University, Karu, Nigeria
Correspondence to: Nehemiah Edem Okon , Biological Sciences Department, Bingham University, Karu, Nigeria.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This study, aimed at the implications of lower and upper respiratory tract infections (LRTI and URTI) in generalized acute respiratory tract infection (ARI) among paediatric patients attending national hospital Abuja, Nigeria, spanned the period of six months, from 1st January to the 30th of June, 2017, at the National Hospital Abuja, located at the central business district of the federal capital territory in Nigeria. Research Method/Design: Retrospectively, a total of 1203 cases of Acute Respiratory Infection seen at National Hospital over the period of six months comprising of 713 (59.3%) males and 490 (40.7%) females were randomly retrieved from the archives. In January, a total number of 202 (16.97%) patients were seen, 17 (11.11%) of them were diagnosed of ARI (having symptoms of both upper and lower respiratory tract infection), 4 (7.40%) were diagnosed of URTI and 13 (18.05%) of them were diagnosed of LRTI. In February a total number of 262 (21.77%), 34 patients (22.22%) were diagnosed of ARI, 4 (5.55%) patients were diagnosed of URTI and 7 (12.96%) patients were diagnosed of LRTI. In March a total number of 231 (19.20%), 41 (26.79%) patients were diagnosed of ARI, 26 patients were diagnosed of URTI and 16 (29.62%) patients were diagnosed of LRTI. In April a total of 171 (14.21%), 20 (13.07%) patients were diagnosed of ARI, 10 (13.88%) patients were diagnosed of URTI and 9 (16.66%) patients were diagnosed of LRTI. In May a total of 175 (14.54%), 21 (13.72%) patients were diagnosed of ARI, 8 (11.11%) patients were diagnosed of URTI and 7 (12.96%) patients were diagnosed of LRTI. In June a total of 162 (13.46%), 20 (13.07%) patients were diagnosed of ARI, 11 (15.27%) patients were diagnosed of URTI and 11 (20.37%) patients were diagnosed of LRTI. We observed generally that the prevalence of Upper Respiratory Tract Infection (URTI) in children between the ages of 0-5 years was far more significant (P<0.05) than Lower Respiratory Tract Infection (LRTI), in children within the same age range, unisex. In conclusion, associated diseases in ARI, such pharyngo- tonsillitis, favoured URTI in higher dimensions and proportions than LRTI that was linked with broncho-pneumonia in this study.
Keywords: Lower Respiratory Tract Infection, (LRTI), Upper respiratory Tract Infection (URTI), Broncho-pneumonia, Pharyngo-Tonsillitis
Cite this paper: Nehemiah Edem Okon , Ajobiewe Helen , Ajobiewe Joseph , Implications of Lower and Upper Respiratory Tract Infections (LRTI and URTI) in Generalized Acute Respiratory Tract Infection (ARI) among Paediatric Patients Attending National Hospital Abuja, Nigeria, American Journal of Medicine and Medical Sciences, Vol. 8 No. 8, 2018, pp. 165-170. doi: 10.5923/j.ajmms.20180808.01.
Article Outline
1. Study Background
- Upper Respiratory Tract Infections (URTI)URTIs are the most common infectious diseases. They include rhinitis (common cold), sinusitis, ear infections, acute pharyngitis or tonsillopharyngitis, epiglottitis, and laryngitis—of which ear infections and pharyngitis cause the more severe complications (deafness and acute rheumatic fever, respectively). The vast majority of URIs have a viral etiology. Rhinoviruses account for 25 to 30 percent of URIs; respiratory syncytial viruses (RSVs), parainfluenza and influenza viruses, human metapneumovirus, and adenoviruses for 25 to 35 percent; corona viruses for 10 percent; and unidentified viruses for the remainder . Because most URIs are self-limiting, their complications are more important than the infections. Acute viral infections predispose children to bacterial infections of the sinuses and middle ear [1], and aspiration of infected secretions and cells can result in LRIs. [1] Acute pharyngitis is caused by viruses in more than 70 percent of cases in young children. Mild pharyngeal redness and swelling and tonsil enlargement are typical. Streptococcal infection is rare in children under five and more common in older children. In countries with crowded living conditions and populations that may have a genetic predisposition, post streptococcal sequelae such as acute rheumatic fever and carditis are common in school-age children but may also occur in those under five. Acute pharyngitis in conjunction with the development of a membrane on the throat is nearly always caused by Corynebacterium diphtheriae in developing countries. However, with the almost universal vaccination of infants with the DTP (diphtheria-tetanus-pertussis) vaccine, diphtheria is rare. Acute ear infection occurs with up to 30 percent of URIs. In developing countries with inadequate medical care, it may lead to perforated eardrums and chronic ear discharge in later childhood and ultimately to hearing impairment or deafness [2]. Chronic ear infection following repeated episodes of acute ear infection is common in developing countries, affecting 2 to 6 percent of school-age children. The associated hearing loss may be disabling and may affect learning. Repeated ear infections may lead to mastoiditis, which in turn may spread infection to the meninges. Mastoiditis and other complications of URIs account for nearly 5 percent of all ARI deaths worldwide [3].Lower Respiratory Tract InfectionsThe common LRIs in children are pneumonia and bronchiolitis. The respiratory rate is a valuable clinical sign for diagnosing acute LRI in children who are coughing and breathing rapidly. The presence of lower chest wall indrawing identifies more severe disease [4, 5]. Currently, the most common causes of viral LRIs are RSVs. They tend to be highly seasonal, unlike parainfluenza viruses, the next most common cause of viral LRIs. The epidemiology of influenza viruses in children in developing countries deserves urgent investigation because safe and effective vaccines are available. Before the effective use of measles vaccine, the measles virus was the most important viral cause of respiratory tract–related morbidity and mortality in children in developing countries. Both bacteria and viruses can cause pneumonia. Bacterial pneumonia is often caused by Streptococcus pneumoniae (pneumococcus) or Haemophilus influenzae, mostly type b (Hib), and occasionally by Staphylococcus aureus or other streptococci. Just 8 to 12 of the many types of pneumococcus cause most cases of bacterial pneumonia, although the specific types may vary between adults and children and between geographic locations. Other pathogens, such as Mycoplasma pneumoniae and Chlamydia pneumoniae, cause atypical pneumonias. Their role as a cause of severe disease in children under five in developing countries is unclear. The burden of LRIs caused by Hib or S. pneumoniae is difficult to determine because current techniques to establish bacterial etiology lack sensitivity and specificity. The results of pharyngeal cultures do not always reveal the pathogen that is the cause of the LRI. Bacterial cultures of lung aspirate specimens are often considered the gold standard, but they are not practical for field application. Vuori-Holopainen and Peltola's (2001) [6] review of several studies indicates that S. pneumoniae and Hib account for 13 to 34 percent and 1.4 to 42.0 percent of bacterial pneumonia, respectively, whereas studies by Adegbola and others (1994) [7], Shann, Gratten, and others (1984) [8], and Wall and others (1986) [9] suggested that Hib accounts for 5 to 11 percent of pneumonia cases. Reduced levels of clinical or radiological pneumonia in clinical trials of a nine-valent pneumococcal conjugate vaccine provide an estimate of the vaccine-preventable disease burden (valency indicates the number of serotypes against which the vaccine provides protection; conjugate refers to conjugation of polysaccharides to a protein backbone). In a study in The Gambia, 37 percent of radiological pneumonia was prevented, reflecting the amount of disease caused by S. pneumoniae, and mortality was reduced by 16 percent [9].Upper respiratory tract colonization with potentially pathogenic organisms and aspiration of the contaminated secretions have been implicated in the pathogenesis of bacterial pneumonia in young children. Infection of the upper respiratory tract with influenza virus or RSVs has been shown to increase the binding of both H. influenzae [10] and S. pneumoniae [11, 12] to lining cells in the nasopharynx. This finding may explain why increased rates of pneumococcal pneumonia parallel influenza and RSV epidemics. A study in South Africa showed that vaccination with a nine-valent pneumococcal conjugate vaccine reduced the incidence of virus-associated pneumonia causing hospitalization by 31 percent, suggesting that pneumococcus plays an important role in the pathogenesis of virus-associated pneumonia [13].Entry of bacteria from the gut, with spread through the bloodstream to the lungs has also been proposed for the pathogenesis of Gram-negative organisms [14], but such bacteria are uncommon etiological agents of pneumonia in immune-competent children. However, in neonates and young infants, Gram-negative pneumonia is not uncommon (Quiambao forthcoming).Viruses are responsible for 40 to 50 percent of infection in infants and children hospitalized for pneumonia in developing countries [15-17] Tupasi and others 1990). Measles virus, RSVs, parainfluenza viruses, influenza type A virus, and adenoviruses are the most important causes of viral pneumonia. Differentiating between viral and bacterial pneumonias radiographically is difficult, partly because the lesions look similar and partly because bacterial superinfection occurs with influenza, measles, and RSV infections [18].In developing countries, the case-fatality rate in children with viral pneumonia ranges from 1.0 to 7.3 percent (John and others 1991; Stensballe, Devasundaram, and Simoes 2003), with bacterial pneumonia from 10 to 14 percent and with mixed viral and bacterial infections from 16 to 18 percent [18, 19]. Bronchiolitis occurs predominantly in the first year of life and with decreasing frequency in the second and third years. The clinical features are rapid breathing and lower chest wall indrawing, fever in one-third of cases, and wheezing [20]. Inflammatory obstruction of the small airways, which leads to hyperinflation of the lungs, and collapse of segments of the lung occur. Because the signs and symptoms are also characteristic of pneumonia, health workers may find differentiating between bronchiolitis and pneumonia difficult. Two features that may help are a definition of the seasonality of RSVs in the locality and the skill to detect wheezing. RSVs are the main cause of bronchiolitis worldwide and can cause up to 70 or 80 percent of LRIs during high season [21, 22]. The recently discovered human metapneumovirus also causes bronchiolitis [23] that is indistinguishable from RSV disease. Other viruses that cause bronchiolitis include parainfluenza virus type 3 and influenza viruses. Even though influenza viruses usually cause URIs in adults, they are increasingly being recognized as an important cause of LRIs in children and perhaps the second most important cause after RSVs of hospitalization of children with an ARI [24]. Although influenza is considered infrequent in developing countries, its epidemiology remains to be investigated thoroughly. The potential burden of influenza as a cause of death in children is unknown. Influenza virus type A may cause seasonal outbreaks, and type B may cause sporadic infection. Recently, avian influenza virus has caused infection, disease, and death in small numbers of individuals, including children, in a few Asian countries. Its potential for emergence in human outbreaks or a pandemic is unknown, but it could have devastating consequences in developing countries [25] and could pose a threat to health worldwide. New strains of type A viruses will almost certainly arise through mutation, as occurred in the case of the Asian and Hong Kong pandemics in the 1950s and 1960s.Other diseases associated with LRTI and URTI are Sepsis, Malaria, Febrile seizures, Pneumonia Diarrhoeal disease, Dehydration, Heart failure, Sickle cell anaemia, Meningitis, Convulsion, (Hamed Esmaili Gourabi, et al, 2012) [26].
2. Methods
- Retrospectively, a total of 1203 cases of Acute Respiratory Infection seen at National Hospital over the period of six months comprising of 713 (59.3%) males and 490 (40.7%) females were randomly retrieved from the archives. The population consisted mainly of patients seen in the Out-patient Paediatric section of National Hospital, Abuja. The researcher was given access to their files to obtain all necessary information needed for the success of this research. The population size and sample size is approximately 1,300 (it could be less). This study was carried out for a period of six months (January to June 2017). The observation method of data collection was used. The sources used to gather all necessary information needed in this research work was mainly secondary data; over the period in which the research work began up until its completion. Hypotheses formulation was used as the instrument of analytical measurement.
3. Research Hypotheses
- H0 – There is no significant difference between the mean percentage prevalence of Acute Respiratory Infection (ARI) and the combined percentage prevalence of Lower respiratory Tract Infection (LRTI) / Upper Respiratory Tract Infection (URTI).Ha – There is significant difference between the mean percentage prevalence of Acute Respiratory Infection (ARI) and the combined percentage prevalence of Lower respiratory Tract Infection (LRTI) / Upper Respiratory Tract Infection (URTI).
4. Result
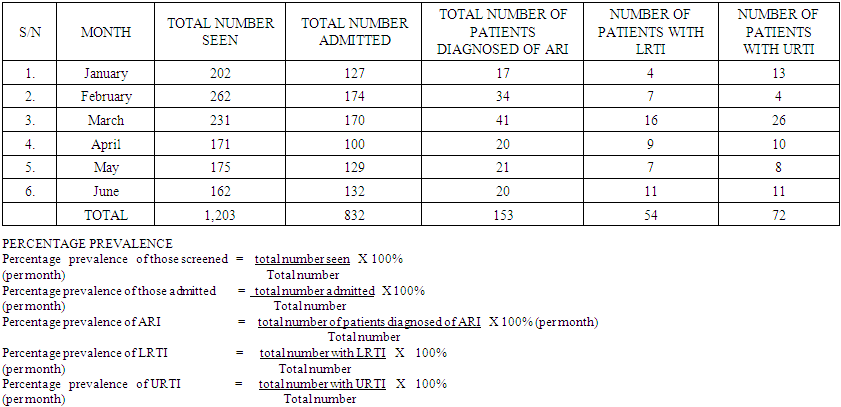 | Table 1. Showing Total No of Patients Seen, Admitted and Diagnosed for ARI, LRTI and URTI |
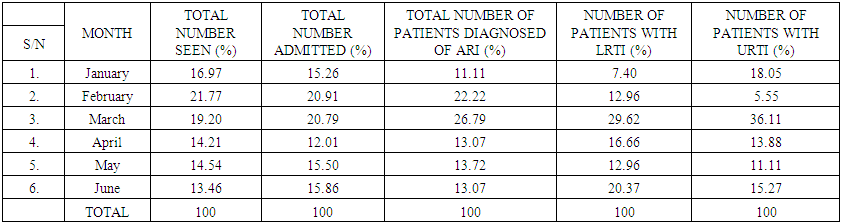 | Table 2. Showing Total Percentage (%) of Patients Seen, Admitted and Diagnosed for ARI, LRTI and URTI |
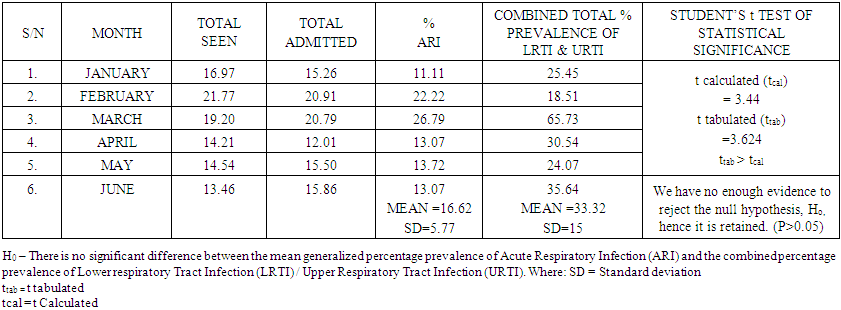 | Table 3. Showing the Inferential Statistical Relationship between ARI, LRTI and UTI |
5. Discussion
- Subjecting the hypotheses to critical analysis revealed the following; we have no enough evidence to reject the null hypothesis, Ho, thus it was retained instead of the alternative/researcher’s hypothesis, Ha. From all the data gathered and analysed, the result H0 – There is no significant difference between the mean generalized percentage prevalence of Acute Respiratory Infection (ARI) and the combined percentage prevalence of Lower respiratory Tract Infection (LRTI) / Upper Respiratory Tract Infection (URTI). ARIs have remained a major contributor to morbidity among children, especially children in developing countries. In the study carried out, a total of 1203 cases of ARIs were seen over a 6-month period (during the harmattan). Most cases of ARI seen in this study were upper respiratory tract infections which is consistent with the reports by other authors. The incidence of pneumonia in developing countries is high due to the increased prevalence of malnutrition, low birth weight and indoor air pollution in developing countries. From the study carried out, there were other diseases found to be associated with generalized Acute Respiratory Tract Infection, these included, Sepsis, Malaria, Diarrhoea, Febrile Convulsion, Pneumonia, Sickle Cell Anaemia, and Meningitis. This agreed perfectly with other similar work done in this area such as the submissions of Eberechukwu et al (2015). Sepsis was the highest with 42 patients (24.45%), followed by Malaria with 34 patients (22.22 %), Dehydration with 20 patients (13.07%), Diarrhoeal disease with 15 patients (9.80%), Febrile Convulsion with 11 patients (7.18%), Pneumonia with 7 patients (4.57%), Sickle Cell Anaemia with 6 patients (3.92%) and meningitis with 1 patient (0.65%). Strikingly, the combined prevalence of Upper Respiratory Tract Infection (URTI) and LRTI are not significantly different from the prevalence of ARI. (P>0.05) in children between the ages of 0-5 years as URTI and LRTI are principal indicators of ARI in children within the same age range, unisex.
6. Conclusions
- In conclusion, associated diseases in ARI, such pharyngo- tonsillitis, favoured both URTI and LRTI in higher dimensions and proportions in this study. Both URTI and LRTI are the determining components of Acute Respiratory Tract Infection whose magnitudes were epitomised by Pharyngo-tonsillitis and broncho-pneumonia respectively. The roles of other diseases highlighted in the discussion above cannot therefore be overruled nor over-emphasized.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML