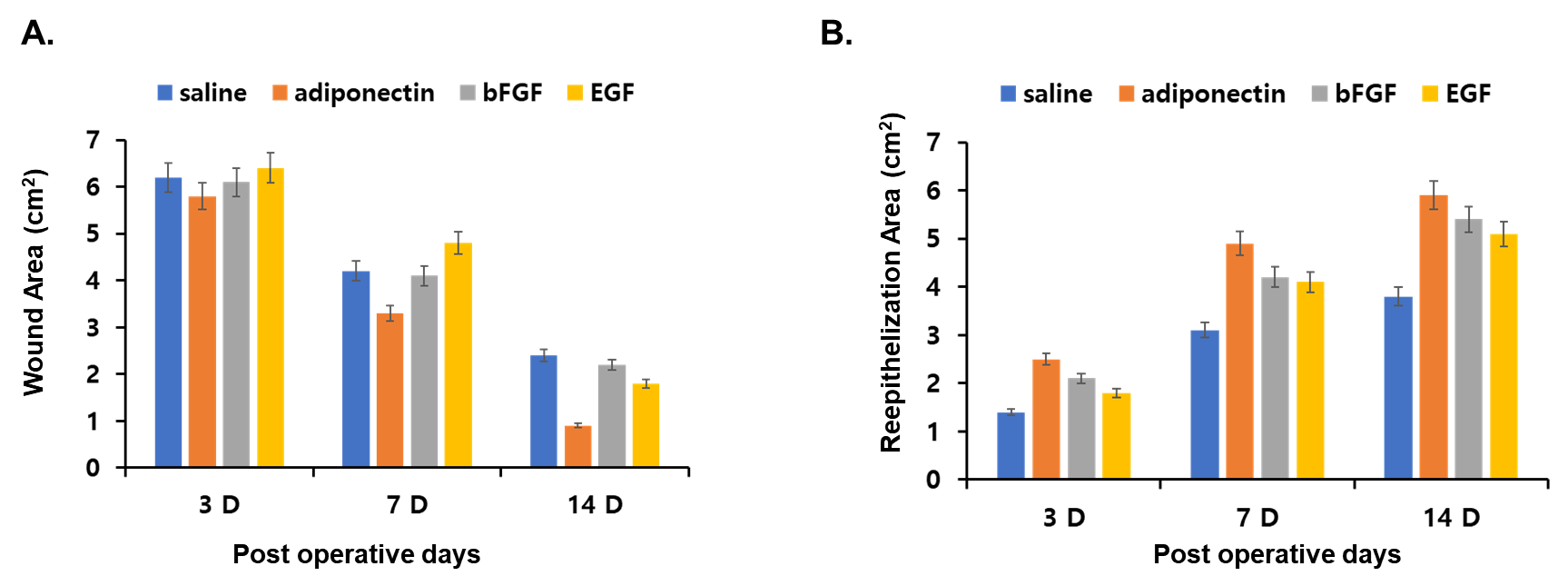-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2018; 8(7): 155-163
doi:10.5923/j.ajmms.20180807.06

Evaluation of Wound Healing Properties of Adiponectin
Ji Soo Yeo1, Young Wook Jo2, 3
1Korea International School (KIS), Gyeonggi, Republic of Korea
2Department of Biotechnology, College of Engineering, Yonsei University, Seoul, Republic of Korea
3Laboratory of Pharmaceuticals, BIOSTANDARD, Seoul, Republic of Korea
Correspondence to: Young Wook Jo, Department of Biotechnology, College of Engineering, Yonsei University, Seoul, Republic of Korea.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The main function of adiponectin has been considered as storage of triglycerides. Adiponectin was considered harmful for healing extensive and deep burns because of poor circulation and easy liquefaction in wound beds, which offer an excellent culture medium for bacteria. However, these traditional concepts have been challenged with the discovery of the endocrine function of adiponectin. In this study, we elucidated the involvement of adiponectin in cutaneous wound healing in vitro and in vivo. Normal human keratinocytes expressed adiponectin receptors, and adiponectin enhanced proliferation and migration of keratinocytes in vitro. This proliferative and migratory effect of adiponectin was mediated via AdipoR1/AdipoR2 and the ERK signaling pathway. To investigate the effects of adiponectin treatment on wound healing, we created full-thickness wounds on each side of the back of male mouse. The wounds were randomly divided to receive normal saline (0.5 mL; controls), adiponectin (0.01 m/kg/cm2), basic fibroblast growth factor (50 U/cm2), and epidermal growth factor (50 U/cm2). Reduction in wound area and wound volume was accelerated with adiponectin treatment as compared with growth factor or control treatment. The thickness of the regenerated epidermis and the number of new vascular nets were markedly increased in adiponectin-treated wounds. Biopsy of adiponectin-treated wounds showed enhanced expression of proliferation cell nuclear antigen (PCNA) and Factor VIII-related antigen, which indicated active cell differentiation and proliferation. Collectively, these results indicate that adiponectin is a potent mediator in the regulation of cutaneous wound healing. We propose that upregulation of systemic and/or local adiponectin levels is a potential and very promising therapeutic approach for dealing with diabetic wounds.
Keywords: Wound Healing
Cite this paper: Ji Soo Yeo, Young Wook Jo, Evaluation of Wound Healing Properties of Adiponectin, American Journal of Medicine and Medical Sciences, Vol. 8 No. 7, 2018, pp. 155-163. doi: 10.5923/j.ajmms.20180807.06.
1. Introduction
- Adiponectin tissue, located beneath the skin, was initially recognized merely as a site of energy storage. However, increasing evidence indicates that adiponectin tissue secretes various bioactive molecules and exerts multiple functions in concert with the epidermis and dermis via endo-, para-, and autocrine pathways [1, 2]. Adiponectin tissue is now considered to be a key participant in the development of skin pathophysiology.Bioactive molecules secreted from adipocytes are termed adipokines. Most adipokines, including TNF-α, IL-6, and resisitin, increase the risk of metabolic syndrome [3]. In contrast, another adipokine, adiponectin, ameliorates insulin resistance and mediates antidiabetic effects in liver and skeletal muscle [4, 5]. Adiponectin-deficient mice exhibit insulin resistance and glucose intolerance. Administration of adiponectin increases glucose uptake and fat oxidation in muscle, reduces glucose production in liver, and improves insulin sensitivity [6]. Thus, adiponectin is a crucial factor in the pathogenesis of diabetes. Adiponectin is present at relatively high concentrations 3–30 μg/ml in circulation, and adiponectin levels are lower in obese and type 2 diabetes patients [7]. Accumulated evidence shows that adiponectin is involved in the various pathological conditions associated with diabetes, such as arterial sclerosis, nephropathy, collateral vessel development, and ocular complications [8-10].Wound healing, a response to injury initiated to reconstruct damaged tissue, involves cell proliferation and migration, matrix protein synthesis and deposition, and tissue remodeling. Cutaneous wound healing requires precise coordination of epithelialization, dermal repair and angiogenesis. [11] Epithelialization ultimately depends on the migratory, proliferative, and differentiation abilities of keratinocytes. The growth and differentiation of keratinocytes is regulated mainly by growth factors, 9 of which the members of the epidermal growth factor (EGF) family and fibroblast growth factor (FGF) family are the most important for skin wound healing. [12]EGF is a polypeptide of 53 amino acids that was first isolated from the mouse submaxillary gland. EGF facilitates epidermal cell regeneration and plays an essential role in the process of wound healing through stimulation of proliferation and migration of keratinocytes. [13] In addition, EGF not only promotes formation of granulation tissue but also stimulates fibroblast motility that induces cell–cell adhesive properties consistent with an epithelial phenotype, thereby enhancing early wound closure. [14]Twenty-two members of FGF are in both humans and mouse. [15] FGF is expressed in dermal and hair follicular cells and can promote growth and differentiation of a broad spectrum of cell types, including dermal fibroblasts, keratinocytes, endothelial cells, and melanocytes and regulate the hair growth and skin regeneration. [16] Because of its powerful mitogenic and angiogenic abilities, it can also influence tissue remodeling, wound healing, and neovascularization. [17]In this study, to investigate the role of adiponectin during the cutaneous wound healing, we first focused on the functions of keratinocytes, key mediators of the re-epithelialization process, and performed functional analyses regarding proliferative and migratory effects. We also examined how adiponectin would be involved in vivo during the wound repair process, using a mouse model of excisional skin wound healing in full-thickness wound of mice. The results of this study indicate that adiponectin enhances proliferation and migration of keratinocytes through AdipoR1/AdipoR2 and the ERK signaling pathway in vitro and that adiponectin regulates the skin wound healing process in vivo by promoting keratinocyte proliferation and migration.
2. Materials and Methods
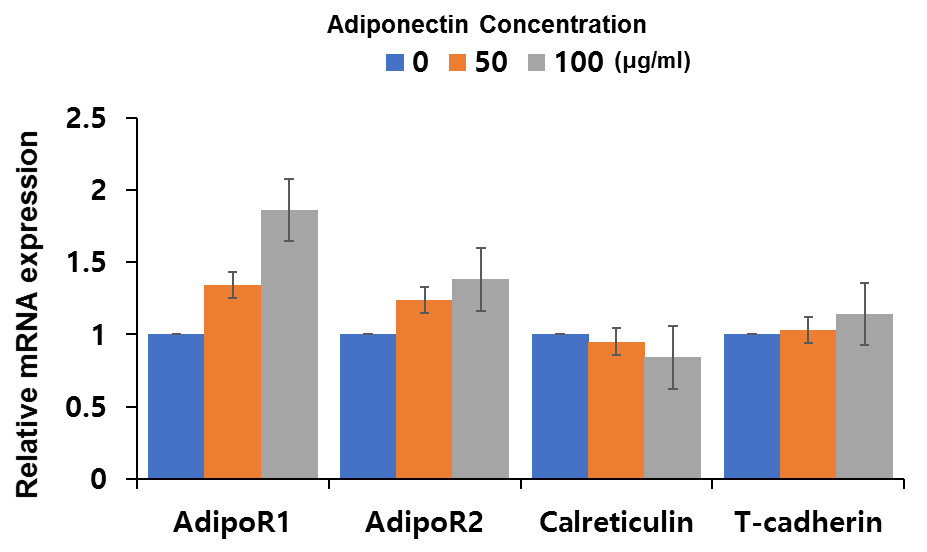
- ReagentRecombinant human adiponectin was isolated and purified as described previously [18]. We verified that purified recombinant adiponectin was free of endotoxin using an endotoxin detection kit (GenScript, Piscataway, NJ). Abs against ERK, p-ERK, p38, p-p38, JNK, p-JNK, Akt, p-Akt, and β-actin were obtained from Cell Signaling Technology (Beverly, MA). Abs against adiponectin, AdipoR1, and AdipoR2 were from Abcam (Cambridge, U.K.). Abs against Ki67 and loricrin were obtained from Novo Castra Laboratories (Newcastle, U.K.) and Covance (Berkeley, CA), respectively. PD98059, U0126, wortmannin, LY294002, SB203580, and SP600125 were also purchased from Cell Signaling Technology.Cell cultureNormal human epidermal keratinocytes were cultured with MCDB153 medium supplemented with insulin (5 μg/ml), hydrocortisone (1 μM), ethanolamine (0.1 mM), phosphoethanolamine (0.1 mM), bovine hypothalamic extract (BHE) (50 μg/ml), and Ca2+ (0.1 mM) as described previously [19, 20]. Third or fourth passage cells were used in this study.Animal model and treatment methodsTen weeks young, healthy, male BL6 mice (25–30 g) were used in this study. The mouse fasted at least 8 hours before surgery and were anesthetized with sodium pentothal (40 mg/kg) intravenously. After clipping the hair on the back of the mouse (about 40×30 cm), the skin was sterilized with 75% alcohol. A sterile template of 3.0 x 2.5 cm was placed on the back, 0.5 cm away from the midback, and a full-thickness wound to the deep fascia corresponding to the template was made by excising the skin. We created wounds from the anterior to the posterior aspect on each side of each animal, for two wounds per animal (Figure 1A). The wounds on each side were randomly divided to receive normal saline (0.5 mL; controls), adiponectin (as described below), basic FGF (bFGF; 50 U/cm2, for 375 U [0.5 mL] for each wound; Torita Bio-Pharma, Zhuhai, China), or EGF (50 U/cm2, 375 U [0.5 mL] for each wound; Torita Bio-Pharma), with 6 wounds in each treatment group. The adiponectin soaking collagen sponge was applied uniformly to the surface of the adiponectin-treatment wounds (0.1mg/kg/cm2 g per wound), and the other wounds were treated as described. Finally, the wounds were covered with one layer of petrolatum gauze and five layers of sterilized dressing stabilized with 3M adhesive tape (3M, St. Paul, MN). Wound area and re-epithelialization areaThe edge of migrating epithelium was easily discernible from the moist granulation tissue, and the epithelial border was at the edge of the healing wound. To measure the wound area, the surface of each wound was covered with a collagen sponge and the edge of the wound was drawn onto the collagen sponge. After being washed and dried, the membrane enclosing the wound area was cut out and weighed. The wound area was calculated as following:Wound area (cm2) = Weight of collagen sponge (g) / Weight of collagen sponge per cm2 The tracing taken immediately after wounding was used as the original area (day 0 area). The re-epithelialization area of the wound on day N was the area between the wound edge and the newly formed epithelial edge on day N. Wounds were considered closed if moist granulation tissue was no longer apparent and the wound appeared to be covered with new epithelium.RT-PCR and quantitative real-time RT-PCR analysisTotal RNA was extracted from cultured keratinocytes and mouse back skin using an RNeasy Mini kit and RNeasy fibrous Tissue Mini kits, respectively (Qiagen, Germantown, MD), respectively. cDNA was synthesized using Superscript III First strand synthesis kits (Invitrogen). For RT-PCR, synthesized cDNA was thermocycled for PCR amplification with 10 μM of each primer and 1.5 U Taq polymerase (Invitrogen). Primers for amplification, and the sizes of respective PCR products were as follows: AdipoR1, 5′-AATTCCTGAGCGCTTCTTTCCT-3′ and 5′-CATAGAAGTGGACAAAGGCTGC-3′; AdipoR2, 5′-TGCAGCCATTATAGTCTCCCAG-3′ and 5′-GAATGATTCCACTCAGGCCTAG-3′ [25]; calreticulin, 5′-AAGTTCTACGGTGACGAGGAG-3′ and 5′-GTCGATGTTCTGCTCATGTTC-3′ [26]; T-cadherin, 5′-GCCACGATCATGATCGATGAC-3′ and 5′-GTCTTCATTTTCCACTTTGA-3′ [27]; and glyceraldehyde-3-phosphate dehydrogenase gene, 5′-TGAAGGTCGGAGTCAACGGATTTGGT-3′ and 5′-CATGTGGGCCATGAGGTCCACCAC-3′ [28]. PCR was performed by 94°C for 5 min, 30 cycles of 94°C for 45 s, 60°C for 45 s, and 72°C for 45 s and a final extension at 72°C for 3 min. The PCR products were analyzed by electrophoresis on 2.5% agarose gels and stained with ethidium bromide, viewed by UV light.For quantitative real-time RT-PCR (qrt-PCR), gene expression was quantified using TaqMan gene expression assay (Applied Biosystems, Warrington, U.K.). PCR conditions were 95°C for 10 min, 40 cycles of 94°C for 15 s, 60°C for 1 min. All samples were analyzed in parallel for glyceraldehyde-3-phosphate dehydrogenase gene expression as an internal control. The relative change in the levels of genes of interest was determined by the 2−△△CT method.Western blot analysisSubconfluent keratinocytes were lysed in lysis buffer containing 20 mM Tris (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM glycerophosphate, 1 mM sodium orthovanadate, 1 mM PMSF, and 1 μg/ml leupeptin. Samples were dissolved in NuPAGE LDS Sample Buffer with NuPAGE Sample Reducing Agent (Invitrogen) and denatured by heating 5 min at 95°C. SDS-PAGE was performed with NuPAGE 4–12% Bis-Tris gels and MES running buffer (Invitrogen). After transfer to an Immobilon-P transfer membrane (Millipore, Bedford, MA), the membrane was incubated for 1 h at room temperature with blocking buffer, overnight at 4°C with the primary Ab, washed, and incubated for 1 h at room temperature with the appropriate secondary Ab. After washing, visualization was performed by ECL (Amersham Biosciences, Buckinghamshire, U.K.). In some experiments, phospho-ERK1/2 levels were quantified by densitometric analysis using ImageJ (available online) and normalized to the total ERK 1/2 levels.Histological assessmentBiopsies taken from each wound bed on days 3, 7, 14, and 21 were fixed in 10% formalin for at least 24 hours, then embedded in paraffin and cut into 5-mm-thick sections using a microtome (Leica, Wetzlar, Germany). For morphological observations, sections were stained with hematoxylin and eosin (H&E), and for immunohistochemical analysis, sections were processed by using a two-step immunostaining kit (Zymed, San Francisco, CA). We stained at least three sections of each sample for the two histological examinations. H&E staining: Tissue sections were deparaffinized in xylene, rehydrated through graded alcohol to phosphate- buffered saline (PBS), stained in H&E, and mounted in resin. Epidermal thickness was measured from stratum basale to stratum corneum at five equidistant interfollicular sites. At least five points were counted on each slide, and the mean thickness of the epidermis was determined. The number of epidermal horns was counted per 1-mm- length epidermis. For immunohistochemical staining, sections were dewaxed and rehydrated, then incubated in 3% H2O2 to block endogenous peroxidase. Sections for Factor VIII-related antigen detection were incubated with 0.4% pepsin at 37 C for 30 minutes, whereas sections for proliferation cell nuclear antigen (PCNA) detection were microwaved for 15 minutes at 95 1C in citrate buffer. Subsequently, sections were incu- bated in rabbit anti-Factor VIII-related antigen and mouse anti-PCNA (clone PC10; both Zymed) monoclonal antibodies at 1:100 dilution, followed by incubation in horseradish peroxidase-conjugated goat anti-rabbit IgG or goat anti-mouse IgG (both Zymed). Sections were finally developed in 3,30-diaminobenzidine, counterstained with hematoxylin, and mounted in resin. PCNA expression was detected in nuclei and Factor VIII-related antigen in cytoplasm. The number of brown-labeled cells was counted to evaluate PCNA labeling and Factor VIII labeling, for a percentage of immunoreactive cells in 100 cells. At least 2,000 cells in three fields of the wounds were counted per slide. The results were assessed by two experienced pathologists blinded to the treatment process. The staining was replicated at least three times.Statistical analysisData analysis involved use of SPSS 10.0 (SPSS, Chicago, IL). Values are expressed as means standard deviation. Differences among groups were analyzed by analysis of variance, followed by t-test. A p < 0.05 was considered significant.
3. Results
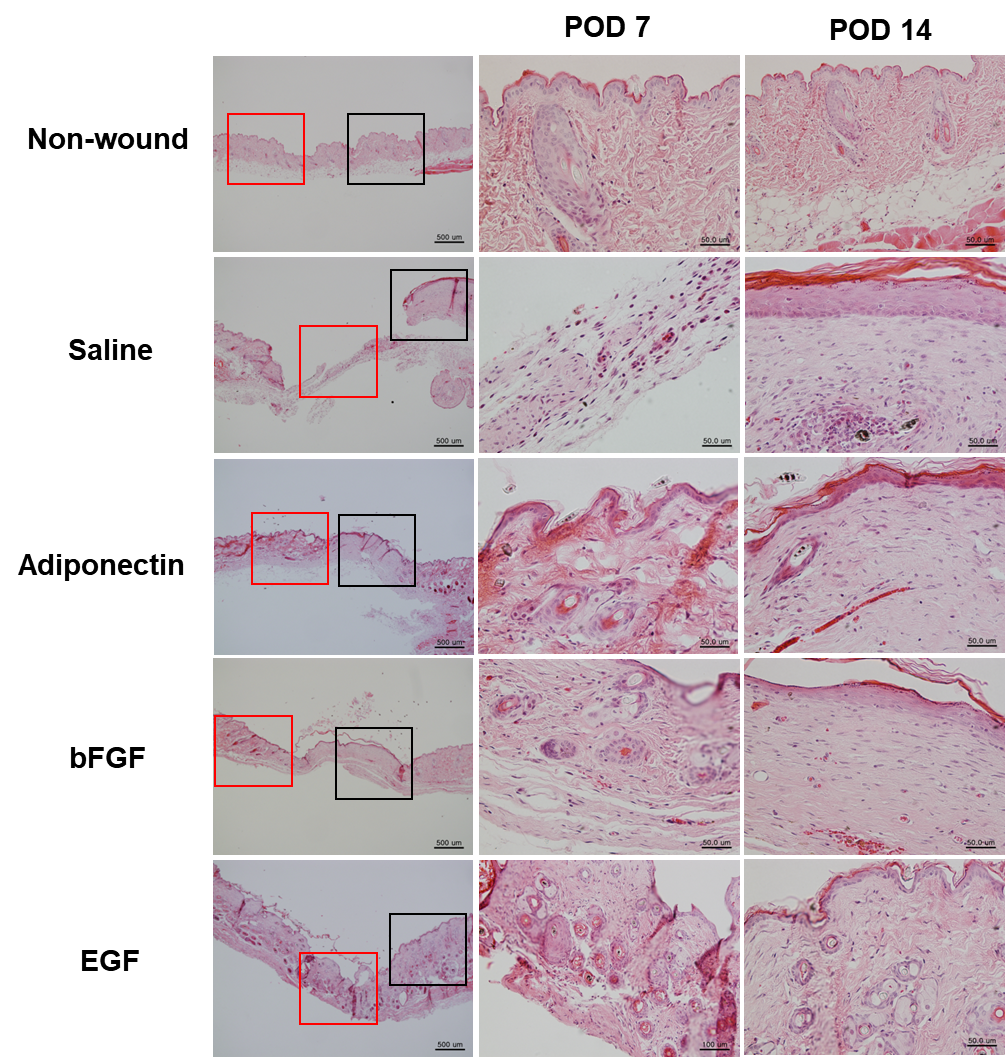
- Effects of adiponectin on wound area and re-epithelialization areaFor the first 3 days, wounds showed no granulation tissue formation or epithelial-tissue growth, but the wounds became smaller. At day 3, all wounds in all groups showed hemorrhage and were filled with granulation tissue. Most of the surface of the granulation tissue was 3–5 mm above the surface of the wounds, and newly formed epithelial growth appeared at the edge of the wounds. Thereafter the surface of the granulation tissue reached the level of the wound margins gradually and the open wound became smaller (Figure 1).
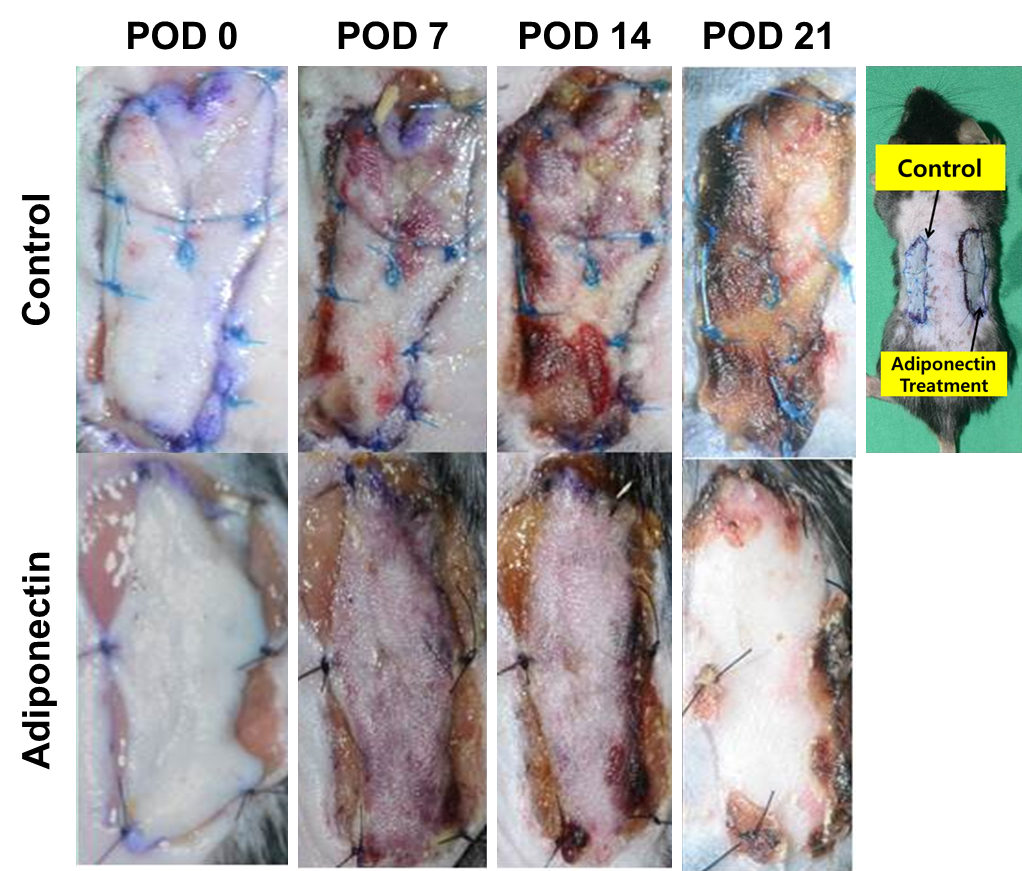 | Figure 1. Models of wounding in mice. Wounds with transplantation immediately after surgery (A) and at days 7 (B), 14 (C), and 21 (D) after transplantation |
4. Discussion
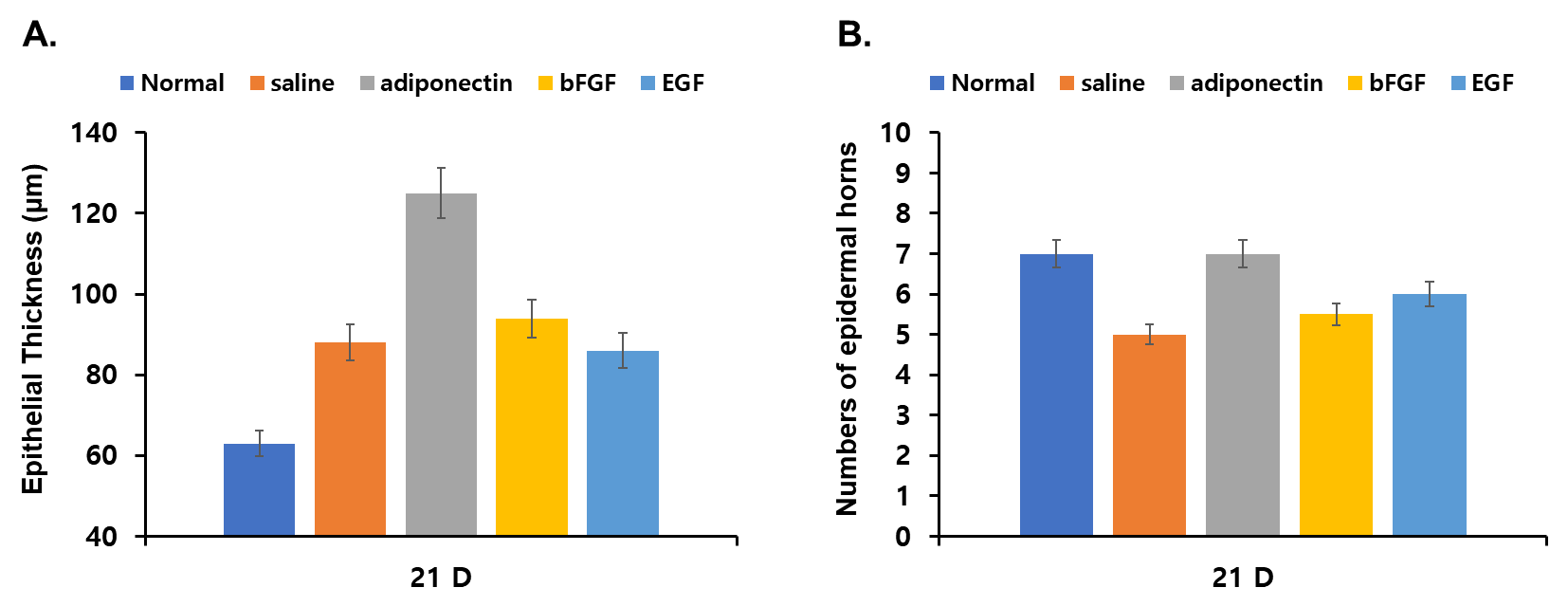
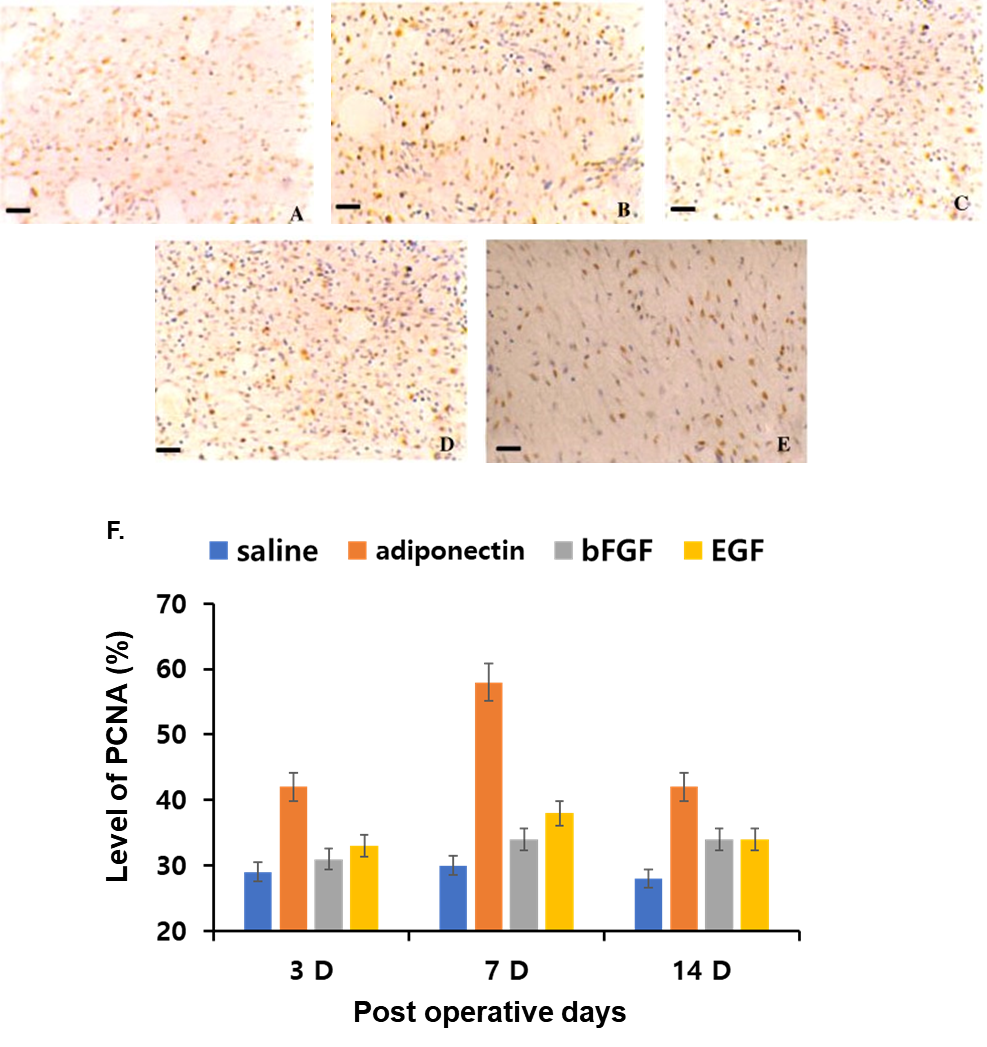
- Adiponectin, a key mediator in the pathogenesis of diabetes, exerts multiple biological activities in various diseases; however, its role in cutaneous wound healing is unknown.In this study, we used a mouse model with full-thickness skin wounds, to study the effects of adiponectin on the time and quality of wound healing. Auto-grafting with adiponectin on full-thickness skin wounds could greatly enhance the production of granulation tissue, as we showed by the reduced volume of wound cavity and by histological evaluation. Wounds treated with adiponectin showed a high number of newly formed vessels. Serial measurement of wound area and histological observation showed accelerated re-epithelialization along the wound edge. Histological analysis and immunohistochemical staining showed that the number of new epidermal horns and the epidermal thickness were significantly increased in wounds treated with adiponectin. The speed and quality of wound healing was enhanced significantly in the wounds treated with adiponectin as compared with wounds treated with growth factors (EGF or bFGF), even though both EGF and bFGF have been shown to stimulate growth of epidermal cells and fibroblasts and play a critical role in accelerating wound healing. [18-25]To clarify this, we investigated pathophysiologic roles of adiponectin during cutaneous wound healing. We demonstrated that keratinocytes express adiponectin receptors and that adiponectin induces proliferation and migration of keratinocytes in a dose-dependent manner through ERK activation in vitro. These in vitro results suggest that adiponetin might contribute to the re-epithelialization phase of optimal wound healing. Although we found beneficial effects of adiponectin in wound healing, the mechanisms underlying the effects should be explored, and some related questions should be answered. First, the mechanisms involved in this process may be related to their endocrine, autocrine/paracrine functions. Hormones, cytokines, and growth factors play critical roles in inflammatory, proliferative and maturation phases of cells during healing, as well as in the remodeling phase, and all wound healing requires growth factors to orchestrate tissue regeneration and re- epithelialization. Adiponectin could be a potential endocrine organ producing some cytokines and growth factors such as leptin, angiotensin II, prostaglandins, TNF-a, and interleukins, 5–7 the main regulators of the inflammatory reaction and local defense in early wound healing. Thereafter, some growth factors related to cell proliferation and differentiation play critical roles in granulation tissue/extracellular matrix formation, and re-epithelialization. The stimulating activity of adiponectin on the proliferation and differentiation of epithelial cells was confirmed in vitro by Sugihara et al. [30] In the current study, the expression of PCNA and Factor VIII was strong in epidermal cells, fibroblasts, and endothelial cells, with the increased formation of granulation tissue and epidermal hyperplasia indicating that these growth factors in adiponectin played a critical role in this stage. [30]As well, adiponectin might be used as an active dressing to cover wounds, creating a good microenvironment to benefit wound healing. Ancient north-american medical treatises have claimed that elk fat is effective for treating burn wounds. [31] With regard to cell proliferation and migration, the behavior of adiponectin seems to depend on cell types. For instance, adiponectin stimulates cell growth in colonic epithelial cells [32], osteoblasts [33], and cardiac fibroblasts [34], where adiponectin is involved in the gastrointenstinal mucosal metabolism, bone metabolism, and myocardial hypertrophy, respectively. Migratory effects of adiponectin have been reported in endothelial progenitor cells [35]. In contrast, adiponectin suppressed cell proliferation and migration in vascular smooth muscle cells [36] and hepatic stellate cells [37], acting as a modulator for vascular remodeling and liver fibrosis, respectively. Most importantly, a certain subset of cell types that are involved in the cutaneous wound healing process exhibit proliferative and migratory behaviors in response to adiponectin. Dermal fibroblasts are the dominant players in the process of granulation tissue formation during cutaneous wound healing. Recent reports have revealed that adiponectin induces proliferation of dermal fibroblasts and upregulation of collagen production [38, 39]. Furthermore, adiponectin induces proliferation of HUVECs and stimulates angiogenesis [40], indicating the positive contribution of adiponectin to optimal wound repair. In addition, adiponectin induces neutrophil migration, which is an initial step of skin wound repair [41].In summary, we propose a potent role for adiponectin, in the regulation of keratinocyte proliferation and migration during cutaneous wound healing and provide a new mechanism underlying delayed wound repair in full-thickness skin wound. On the basis of our data, we propose that local upregulation of adiponectin levels may represent a novel therapeutic strategy for skin wounds healing.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML