-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2018; 8(7): 137-144
doi:10.5923/j.ajmms.20180807.04

Ozone Preconditioning Attenuates Spinal Cord Ischemia/ Reperfusion Injury in Rats
Dalia El Agamy1, Asmaa Mohamed2
1Department of Physiology, Faculty of Medicine, Menoufia University, Egypt
2Department of Pathology, Faculty of Medicine, Menoufia University, Egypt
Correspondence to: Dalia El Agamy, Department of Physiology, Faculty of Medicine, Menoufia University, Egypt.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Spinal cord ischemia/reperfusion injury (SC-IRI) can result in paraplegia. In this study, we investigated the protective effect of medical ozone on SC-IRI. To investigate this, 40 rats were divided into five equal groups: the control (n=8), sham (n=8), ozone (1 mg/Kg, n=8), SC-IRI (n=8), and ozone-IRI (ozone + SC-IRI) groups. After evaluation of neurologic function by using Tarlov scores (48 hours after ischemia), serum levels of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) were determined. Spinal cords were immediately removed for determination of malondialdehyde (MDA), prostaglandin E2 (PGE2), heat shock protein 70 (HSP70) levels, and SOD and GSH-Px activities. Hematoxylin-eosin–stained (H&E) spinal cord sections were done for histologic study. The neurologic outcomes in ozone-IRI group were better than that of SC-IRI group (P=0.001). Ozone-preconditioning reduced serum levels of TNF-α and IL-6. Significantly decreased levels of MDA, PGE2, and HSP70 in spinal cord homogenate of ozone-IRI group were observed. Activities of SOD and GSH-Px were increased in ozone-IRI group when compared to SC-IRI group. Histologic analysis in rats of ozone-IRI group showed markedly reduced neuronal cell injury. Our results suggest that ozone preconditioning may protect spinal cord neurons from SC-IRI due to its antioxidative and anti-inflammatory properties.
Keywords: Spinal cord, Ischemia/reperfusion injury, Ozone, Heat shock protein 70
Cite this paper: Dalia El Agamy, Asmaa Mohamed, Ozone Preconditioning Attenuates Spinal Cord Ischemia/ Reperfusion Injury in Rats, American Journal of Medicine and Medical Sciences, Vol. 8 No. 7, 2018, pp. 137-144. doi: 10.5923/j.ajmms.20180807.04.
Article Outline
1. Introduction
- Ischemia/reperfusion injury (IRI) is a serious complication in many surgical interventions, such as aortic cross clamping, organ transplantation, and cardiopulmonary bypass [1]. Paraplegia remains a devastating complication for SC-IRI that occurs in patients undergoing thoraco-abdominal aortic surgery; up to 40% of patients develop paraplegia within the first 24 postoperative hours [2]. This can be attributed to ischemia and interruption of blood supply; however the major proportion of injury occurs during reperfusion due to high metabolic activity and free radicals release that consume the available buffer enzymes, and loss of cellular ATP that inhibits the membrane pumps and results in intracellular hyperkalemia with subsequent cellular edema and acidosis, and probably apoptosis and cell death [3].Injury of the central or peripheral nervous system is accompanied by up or downregulation of a number of molecules for mediation of nerve repair or augmentation of the damage [4]. Heat shock proteins are chaperone proteins that are up regulated when cells are subjected to stress and injury. They are called protein guardians because they repair partially damaged proteins, and prevent proteins aggregation and denaturation promoting cell survival after stress or injury [5, 6]. HSP70 is the most frequently produced protein after spinal cord injury with a significant anti-inflammatory and cytoprotective actions [7].Ozone is a molecule that is formed of 3 oxygen molecules. Medical ozone consists of a mixture of ozone and oxygen (O3/O2) [8]. Ozone therapy has been widely used in medical practice such as chronic ischemic diseases, peritonitis, chronic cutaneous ulcers, infected wounds, burns, gangrenes, and joint problems. Ozone oxidative preconditioning provides a prophylactic approach for minimizing organ damage. It creates an alarm reaction in the body, upregulates antioxidant enzyme capacity and anti-inflammatory pathways [9] and induces adaptation and tolerance to oxidative stress preparing the body to face the pathophysiologic mechanisms mediated by free radicals [10]. It also increases blood flow to the tissues, enhances the general metabolism, and promotes growth factors release from platelets [11]. Hence, we aimed in this study to investigate the neuroprotective effect of ozone preconditioning on spinal cord in IRI rat model and the effect on HSP70 was also determined.
2. Materials and Methods
2.1. Animals and Experimental Design
- Forty adult male albino rats weighing 200-250 g were used in this study. Animals were kept at room temperature, 26 ± 1°C, in animal house of Menoufia Faculty of Medicine, fed with standard laboratory chow and water ad libitum, and maintained with artificial light/dark cycle of 12h. Rats were randomly assigned into 5 groups as the following:Control rats (n=8): rats underwent no surgery, ozone rats (n=8): O3/O2 mixture (Longevity Ozone generator EXT 120, Canada) was administered intraperitoneally (IP) in a dose of 1mg/kg [9]. Sham rats (n=8): rats were subjected to laparotomy without aortic clampling, SC-IRI rats (n=8): rats were subjected to laparotomy and clamping of the aorta just above the bifurcation by non-traumatic vascular clamp for 45 minutes followed by reperfusion for 48 hours. Ozone-IRI rats: the IRI procedure was repeated in the ozone-IRI rats, but 1 mg/kg O3/O2 mixture was administered IP prior to the procedure by 2 hours [9]. The experiments were performed in accordance with the ethical guidelines for the care and investigations in laboratory animals and were approved by the ethical committee of the Faculty of Medicine, Menoufia University.
2.2. Surgery Procedure
- Spinal cord IRI was performed as previously described [12]. In brief, rats were initially anesthetized by intramuscular ketamine (50 mg/Kg) and then by half dose of ketamine when required. The abdominal aorta was exposed through mid line laparotomy and clamped by a non-traumatic vascular clamp which was placed above the aortic bifurcation and under the left renal vein. Rectal temperature was monitored and maintained at 37±0.5°C by a heat lamp. Distal and proximal aortic pressures were monitored by using 2 catheters inserted into the tail artery and the left carotid artery respectively. 0.9% NaCl was infused during the procedure and 10 mg/Kg cefazolin was injected intravenously immediately before the surgery to prevent infection. The aortic clamp was released after 45 minutes and the wound was closed. Animals were allowed to recover for 3 hours at 28°C in a plastic box. Then, they were placed in their cages with free access to food and water.
2.3. Neurological Assessment
- Neurological assessment was done by investigators blinded to the experimental processes 48 hours after surgical procedure. Neurological function of hind limb was assessed using Tarlov Scoring System: 0= no voluntary movement of the hind limb, 1= perceptible movement of joints, 2= active movement but cannot sit without assistance, 3= able to sit but cannot hop, 4=weak hop, 5= excellent motor function of the hind limb [13].
2.4. Blood Sampling and Biochemical Assays
- Retro-orbital blood samples were collected from each rat after neurological assessment via heparinized microcapillary tubes and the blood was allowed to clot for 30 minutes at room temperature, then, centrifuged at 2000 rpm for 10 min to separate serum samples. Serum samples were stored at -20°C. Serum samples were used for determination of TNF-α and IL-6.
2.4.1. Determination of Serum TNF-α and IL-6 Levels
- Serum levels of TNF-α and IL-6 (Quantikine® ELISA, R&D Systems Inc., MN, USA) were determined by enzyme linked immunosorbent assay (ELISA) technique using an automatic optical reader (SUNRISE Touchscreen, TECHAN, Salzburg, Austria) [14, 15].
2.5. Preparation of Spinal Cord Homogenate
- After collection of blood samples, animals were sacrificed using a lethal dose of thiopental sodium injection [13]. The lumbar enlargement of spinal cord (L3 – L5) was rapidly removed and divided into two equal parts. A section in the cranial portion of tissue was dissected out, sliced into small pieces, and homogenized using an Omni tissue homogenizer. Homogenization was done by a tube pestle in ice-cold lyses buffer [0.1 M potassium phosphate, pH 7.4, 1 mM EDTA, 10 μM indomethacin (Cayman Chemical, Ann Arbor, MI, USA)] and acetone was added (2x sample volume). Samples were then centrifuged at 1500 x g for 10 minutes at 4°C and the supernatants were stored at – 80°C for further use [16]. The caudal portion of the tissue was fixed in 4% paraformaldehyde/phosphate buffered saline followed by paraffin-embedding for sectioning.
2.5.1. Determination of Spinal Cord Homogenate Levels of MDA, and SOD and GSH-Px Activities
- Tissue levels of MDA (QuantiChrom™, BioAssay Systems, USA) glutathione peroxidase (GSH-Px) and superoxide dismutase (SOD) (EnzyChrom™, BioAssay Systems, USA), were determined by colorimetric method [17-19].
2.5.2. Estimation of Spinal Cord Homogenate Level of PGE2 and HSP70
- Enzyme LISA kits were used for estimation of PGE2 (DRG International, Inc, USA) and HSP70 levels in spinal cord homogenate (MyBioSource, Inc., USA) according to the manufacturer’s instructions and measurement was performed with ELISA automatic optical reader and the absorbance was taken at 450 nm (SUNRISE Touchscreen, TECHAN, Salzburg, Austria).
2.6. Histological Analysis
- Serial 5-µm thick coronal sections were collected from paraffin embedded portion of the spinal cord followed by H&E staining to assess histological changes. Slides were analyzed for the number of viable neurons under light microscope by a pathologist blinded to the groups [20].
2.7. Chemicals
- Ozone mixture was obtained from ozone generator from ozone unit, Faculty of Medicine, Menoufia University.
2.8. Statistical Analysis
- Data were expressed as mean ± SD. Testing significance was performed using one-way analysis of variance (ANOVA). Post-hoc Tukey’s test was applied to identify the source of statistical significance. P-value < 0.05 was considered statistically significant.
3. Results
- Both the sham and the ozone groups did not show any significant changes in all measured parameters when they were compared with the control group and with each other (P> 0.05).
3.1. Motor Function of Hind Limb
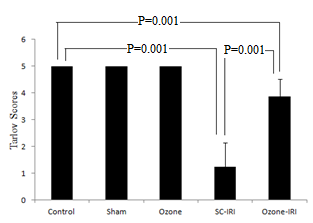
- The sham and ozone-treated rats showed normal motor function of lower limbs throughout the observation period. Equivalent Tarlov scores were observed between both groups and control group (P =1.0). A 45-min aortic occlusion followed by 48 hours of reperfusion resulted in severe lower limbs neurologic deficits as indicated by the significantly lower Tarlov scores of the SC-IRI group when compared to control group (P = 0.001). Whereas the ozone preconditioning remarkably enhanced the motor function of the lower limbs after SC-IRI as indicated by significantly higher Tarlov scores in Ozone-IRI group when compared to SC-IRI group (P = 0.001) but the scores were still significantly lower than control (P = 0.001) “Figure 1”.
3.2. Serum Level of TNF-α and IL-6
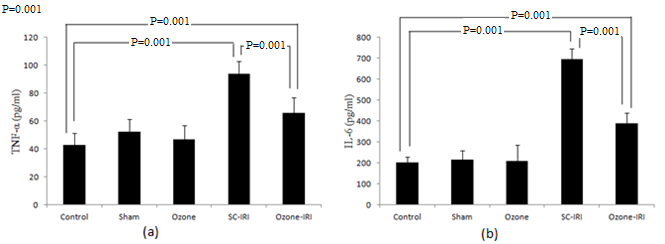
- Ischemic RI group showed significant increase in serum levels of both TNF-α and IL-6 when compared to control group (P=0.001), and their levels were significantly decreased in ozone-IRI group when compared to SC-IRI group (P=0.001), but still significantly higher than control group (P=0.001) “Figure 2”.
3.3. Spinal Cord Homogenate Level of MDA, and Activities of SOD and GSH-Px
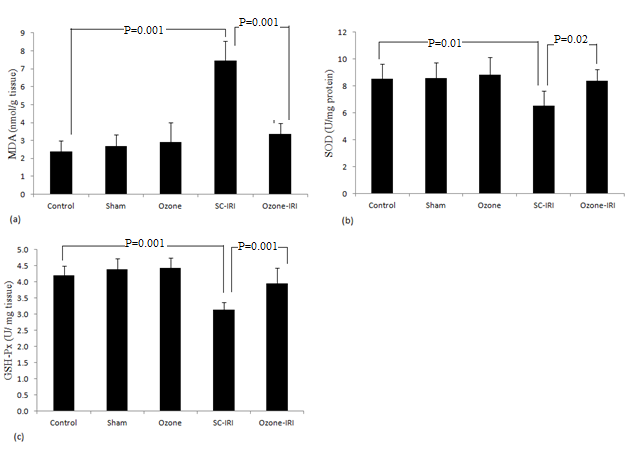
- Significant elevation was observed in the level of MDA in the SC-IRI group when compared to the control groups (P= 0.001). MDA was significantly decreased in ozone-IRI group when compared to SC-IRI group (P= 0.001) to a level that was insignificantly changed from control groups (P= 0.1). GLUT-Px and SOD activities were significantly decreased in the SC-IRI group when compared to the control groups (P= 0.01 and P= 0.001 respectively), and their activities were significantly increased in the ozone-IRI group when compared to the SC-IRI group (P= 0.02 and P= 0.001) to a level that was insignificantly changed from the control “Figure 3”.
3.4. Spinal Cord Homogenate Level of PGE2 and HSP70
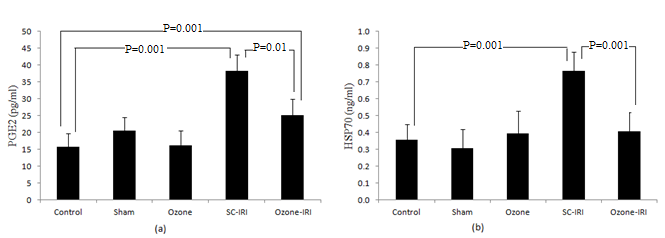
- Levels of both PGE2 and HSP70 in SC-IRI group was significantly higher than that in control groups (P=0.001). Ozone preconditioning significantly decreased PGE2 in ozone-IRI group to a level that was significantly lower than that in SC-IRI group (P=0.001) and significantly higher than that in control groups (P=0.01). Level of HSP70 was significantly decreased in ozone-IRI group when compared to SC-IRI group (P=0.001) to a level insignificantly changed from control groups (P=0.9) “Figure 4”.
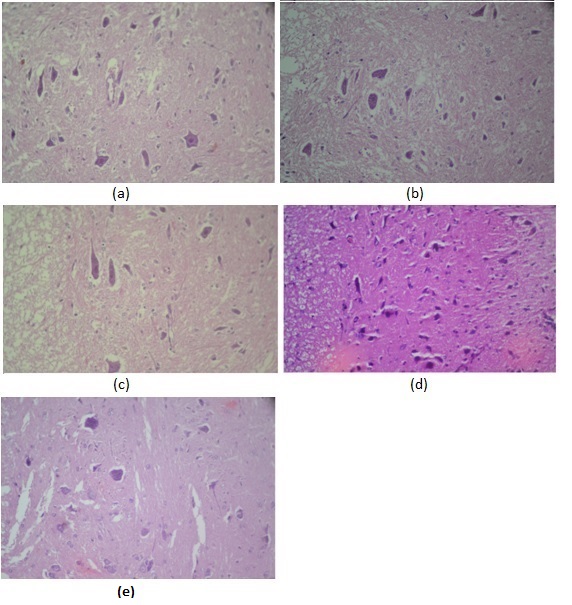
3.5. Histologic Evaluation
- Decreased neuronal viability was observed after ischemia and reperfusion with no ozone preconditioning “Figure 5”. Qualitatively, sham and ozone rats showed increased neuron viability. Rats exposed to IRI, including ozone-preconditioned rats, had frequent degenerated necrotic neuronal bodies relative to control rats. Spinal cord sections from SC-IRI group showed severe neuronal injury, most of neuronal cells showed pyknotic shrunken nuclei, invisible nucleoli, and deep eosinophilic cytoplasm. Neurons in the ozone-IRI rats, however, had more viable neuronal cells with abundant cytoplasm, centrally located nuclei and prominent nucleoli. In addition, inflammatory cells were increased in SC-IRI group relative to control, sham and ozone groups.
4. Discussion
- The aim of this work was to determine the effect of ozone preconditioning on SC-IRI and to investigate the possible involvement of HSP70 in the pathogenesis of injury. Findings from the present study showed that ozone preconditioning attenuated spinal cord injury, after 45 minutes aortic ischemia followed by 48 hours of reperfusion, and preserved the motor function of the extremity. That was associated with a decrease in HSP70, inflammatory mediators, such as TNF-α, IL-6, and PGE2, and oxidative stress markers such as MDA, SOD, and GSH-Px.Spinal cord injury following repair of thoracic aortic aneurysm and other disorders of the thoracic aorta continues to be an unpredictable and challenging complication. The pathophysiology of SC-IRI is complex and not fully understood [21]. The main cause of SC-IRI may be tissue hypoperfusion which causes marked dysfunctional injury and neurological damage that is aggravated by reperfusion [22]. The consequences of ischemia and reperfusion on neuronal death are complex; while reperfusion limits the extent of necrosis, it may also results in deleterious effects that are described as reperfusion injury. Several mechanisms have been emerged such as acute oxidative stress, inflammatory response, and the release of immune mediators [23]. Ischemia induces expression of HSP70 which can modulate inflammatory responses. It can be proinflammatory and it may also have anti-inflammatory and cytoprotective effects [2].In the present study we induced spinal cord ischemia by clamping of abdominal aorta for 45 minutes followed by 48 hours of reperfusion. Spinal cord injury was observed, evidenced by marked hind limb motor dysfunction and histological changes. We observed significant increase in spinal cord MDA level that was associated with significant decrease in SOD and GSH-Px activities. IRI results in excess generation of free radicals causing lipid peroxidation and oxidative damage. Overproduction of free radicals leads to inactivation of antioxidant enzymes, consumption of antioxidants, and failure of the antioxidant defense mechanism to protect the tissue from oxidative damage [24]. Consistent with our results, several previous studies reported that IRI resulted in significant increase in lipid peroxidation and decrease in tissue SOD, GSH, and catalase levels when compared to control group [22, 25]. Whereas other studies reported increased spinal cord tissue activity of SOD and catalase [26, 27] which could be explained by different time points of assays, as Song et al. [27] observed significant increase at enzyme activities approximately 30 minutes and 6 hours after reperfusion. Spinal cord IRI includes two phases, acute ischemic and delayed reperfusion injury. Inflammation is a major contributor to both phases. Inflammatory cytokines such as TNF-α, IL-6, IL-8, IL-1, IL-10 are expressed by inflammatory cells [28]. Several previous studies, consistent with our results, reported enhanced release of TNF-α, IL-6, and PGE2 in spinal cord IRI rat models [22, 29, 30]. Ischemia induces inducible nitric oxide synthase enzyme activity with subsequent overproduction of nitric oxide that leads to activation of cyclo-oxygenase-2 (COX-2) and overproduction of PGE2 which participate in ischemic injury in the human brain [31]. Severe oxidative stress induces nuclear transcriptional factor kappa B (NFκB) which produces an inflammatory response via production of COX2, PGE2, and cytokines [32]. The destructive activity of inflammatory mediators and oxygen free radicals on spinal cord tissue is thought to be one of the causes of tissue necrosis and paraplegia in SC- IRI [24, 33].Results from the present study showed that 48 hours after IRI, HSP70 was significantly higher in the spinal cord homogenate of the SC-IRI group than control groups. The intracellular inducible HSP70 is induced by ischemia [34]. It protects cells from proteotoxic stress, prevents protein denaturation and aggregation, and inhibits cell death and apoptosis [2]. It is the most frequently produced protein after SC-IRI [7]. HSP70 can be used to recognize neuronal injury and assess the effectiveness of some prevention and treatment methods for central nervous system injury [33]. Preconditioning is a repeated and controlled stress that provides protection against a prolonged and severe stress. It includes oxidative preconditioning, ischemic preconditioning, thermal preconditioning, and chemical preconditioning [35]. Preconditioning includes an early phase which becomes manifest within minutes, and a late phase which becomes manifest within approximately 12 hours and lasts for at least 72 hours. The late phase is powerful and results in approximately 50% reduction in total dysfunction. The two phases have different pathophysiology and different mechanisms [36]. In the present study, we studied the effect of ozone preconditioning after 48 hours of IRI. Ozone oxidative preconditioning protects cells through reduction of oxidative stress and apoptosis. It was able to reduce the damage to spinal cord of IRI that was verified by biochemical, histological, and neurological observations. There was decreased spinal cord homogenate level of MDA and enhanced SOD and GSH-Px activities. Consistent with our results, ozone preconditioning increases antioxidant capacity, attenuates mitochondrial damage, and protects the myocardium against IRI [37]. Also, ozone preconditioning attenuates renal dysfunction, histological damage, and renal oxidative stress in renal IRI rat model [38]. It also increases the activity of antioxidant enzymes such as SOD and GSH-Px and decreases MDA in a rat model of kidney transplantation [39]. Other study reported that ozone preconditioning protects against hepatic IRI by adenosine accumulation and by blocking xanthine/xanthine oxidase pathway, reducing generation of reactive oxygen species (ROS) [40]. Application of IP ozone enhanced GSH-Px that is involved in detoxification of lipid peroxides [35]. We also observed significant inhibitory effect of ozone preconditioning on TNF-α, IL-6, and PGE2 production. Ozone preconditioning had potent anti-inflammatory properties via modulation of toll-like receptors 4- NF-κB pathway [41]. Intraperitoneal O3/O2 mixture injection modulates phagocytic activity by a direct effect on peritoneal macrophages, inhibits prostaglandin synthesis, releases bradykinins, and increase IL-8 which activates NF-κB, allowing production of ROS scavengers [42]. The anti-inflammatory effect may also be a consequence of stimulation of antioxidant defenses as oxidative stress is strongly involved in induction of the inflammatory process [35]. Some molecules are implicated in late preconditioning such as heat shock proteins, heme oxygenase-1, and nitric oxide. Late preconditioning is evident 24 hours after the application of a preconditioning and lasts for 2 – 4 days. The stimulation of heat shock proteins, especially HSP70, induces tolerance to reperfusion damage due to binding to apoptotic factors and preventing activation of caspase [43]. In spinal cord samples from rats exposed to ozone preconditioning, a significant reduction of HSP70 was detected suggesting a regulatory role of ozone preconditioning on expression of HSP70. Ozone therapy also activates nuclear factor-erythroid 2-related factor 2 (Nrf2) via moderate oxidative stress. Nrf2, via induction of antioxidant response elements transcription, activates the production of numerous antioxidant enzymes such as SOD, GSH-Px, glutathione-s-transferase, heme-oxygenase-1, catalase, and heat shock proteins [32].
5. Conclusions
- We demonstrated enhanced inflammatory mediators, oxidative stress and HSP70 expression in spinal cord during IRI injury. The present study supports the finding that ozone preconditioning has a partial protective effect against SC-IRI. The oxidative ozone preconditioning improves the motor function and reduces the level of oxidative stress, HSP70 and inflammatory mediators.
ACKNOWLEDGEMENTS
- Thanks to all staff members of physiology department and technicians of the Central Lab of Collage of Medicine, Menoufiya University, Egypt for their great help in every step in this work.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML