-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2018; 8(6): 117-122
doi:10.5923/j.ajmms.20180806.04

Characteristics of Molecular Breast Cancer Subtypes among Uzbekistan’s Women
Miryusupova G. F.
Tashkent City Oncology Center, Tashkent Pediatric Medical Institute, Uzbekistan
Correspondence to: Miryusupova G. F., Tashkent City Oncology Center, Tashkent Pediatric Medical Institute, Uzbekistan.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In the Republic of Uzbekistan biological characteristics of breast cancer (BC) are published in fragments. In this cohort study included 912 breast cancer patients. Material: Date from Tashkent City Cancer Registry about female BC, diagnosed in 2008-2018 was analyzed. Cohort study included 2 groups of BC: the 1 group of BC is Uzbek native women 500cases, the 2 group-412 cases. Results: Median ageof BC in the 1 group is 48,5±11 years, 2 group is 52±11,4 years. Conclusions: Our study findings suggest that the prevalence of triple negative (21,6%) breast cancer in Uzbekistan’s.
Keywords: Breast cancer, Uzbekistan’s women, Biological subtypes, Comorbidity diseases
Cite this paper: Miryusupova G. F., Characteristics of Molecular Breast Cancer Subtypes among Uzbekistan’s Women, American Journal of Medicine and Medical Sciences, Vol. 8 No. 6, 2018, pp. 117-122. doi: 10.5923/j.ajmms.20180806.04.
1. Introduction
- An interest to the heterogeneity of breast cancer remains an important issue. Breast cancer is the most frequently diagnosed cancer and the leading cause of cancer death in females worldwide, accounting for 23% (1.38 million) of the total new cancer cases and 14% (458,400) of the total cancer deaths in 2008 [14, 28]. About half the breast cancer cases and 60% of the deaths are estimated to occur in economically developing countries. In general, incidence rates are high in Western and Northern Europe, Australia/New Zealand, and North America; intermediate in South America, the Caribbean, and Northern Africa; and low in sub-Saharan Africa and Asia. The factors that contribute to the international variation in incidence rates largely stem from differences in reproductive and hormonal factors and the availability of early detection services [5, 11]. Reproductive factors that increase risk include a long menstrual history, null parity, recent use of postmenopausal hormone therapy or oral contraceptives, and late age at first birth [7]. Alcohol consumption also increases the risk of breast cancer [3, 9]. In many African and Asian countries however, including Uganda, South Korea, and India, incidence and mortality rates have been rising [12, 13], with changes in reproductive patterns, physical inactivity, and obesity being the main contributory factors [5, 4, 8]; increases in breast cancer awareness and screening activity may be partially responsible for the rising incidence in these populations. Maintaining a healthy body weight, increasing physical activity, and minimizing alcohol intake are the best available strategies to reduce the risk of developing breast cancer [10]. According to the Centers for Disease Control and Prevention (CDC), 230.815 women and 2.109 men in the USA were diagnosed with breast cancer in 2013. In the same year, some 40.860 women and 464 men in the USA died from breast cancer. With the exception of skin cancer, breast cancer in the U.S. is the most common cancer in women across all races. However, the rates per 100,000 women differ greatly among certain races and ethnicities: rates per 100,000 women in U.S.: all races: 123.7, white: 124.4, black: 122.9, Hispanic: 92.5, Asian and Pacific Islander: 91.1, American Indian and Alaska Native: 72.3.The differences among different races could be due in part to reproductive patterns. For instance, white women are more likely to put off childbirth longer and to have fewer children overall. The average body weight of certain ethnicities and the use of menopause hormone therapy may also play a role in these different incident rates [25, 26, 29, 30].Early detection through mammography has been shown to increase treatment options and save lives, although this approach is cost prohibitive and not feasible in most economically developing countries [1, 27]. Recommended early detection strategies in these countries include the promotion of awareness of early signs and symptoms and screening by clinical breast examination [2].Over the last decade, gene expression analysis has been extensively submitted in breast cancer research, aiming to elucidate the molecular bases underlying biological features (histological grade, metastasis ability), also finding the specific patterns associated with prognostic and therapy response. Moreover delineating the 5 subtypes (luminal A, luminal B, HER2, basal-like, normal-like) resembled to gene expression pattern confirmed - at molecular level - the subsisting concept of breast cancer clinical and morphological heterogeneity.The epidemiology of BC has been extensively studied in developed countries; however, epidemiological data is scarce in the Central Asia. Breast cancer ranks the first place in the structure of morbidity and mortality from malignant tumors of the female population of the Republic of Uzbekistan (Fig.1, 2). It is 2892 new cases of BC in 2015 (Fig. 3). However molecular classification and its prognostic significance in Uzbekistan women with breast cancers have not been studied.
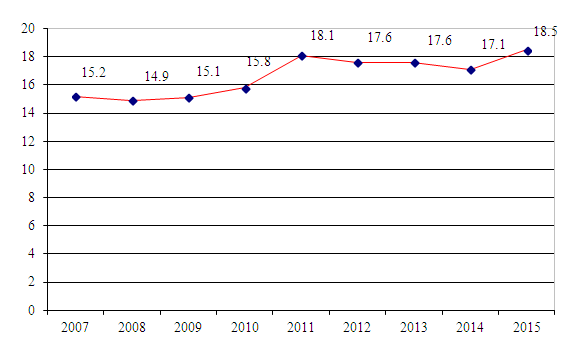 | Figure 1. Estimated Age-Standardized Incidence Rates Per 100 000 by Uzbekistan (2007-2015) |
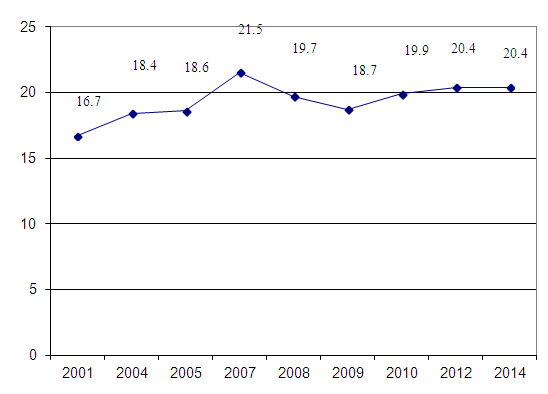 | Figure 2. Estimated Mortality Rates Per 100 000 by Uzbekistan (2001-2014) |
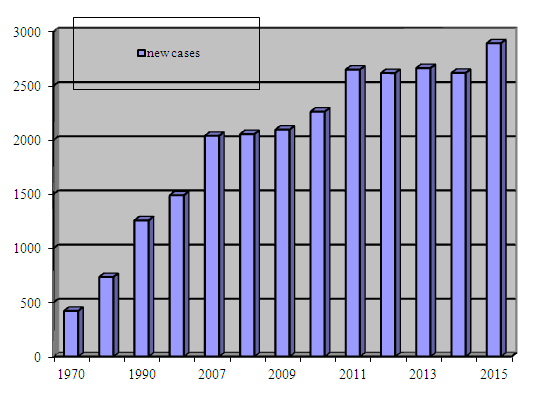 | Figure 3. New breast cancer cases in Uzbekistan (1970-2015) |
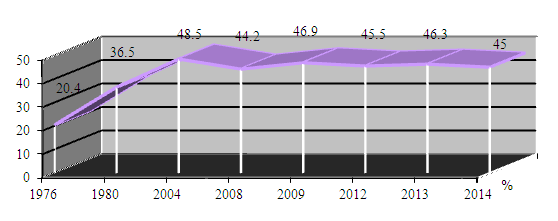 | Figure 4. Five-year survival (females only) 1976-2014, the Republic of Uzbekistan |
2. Methods
- Date from Tashkent City Cancer Registry about female BC, diagnosed in 2008-2018 was analyzed. Estrogen receptor (ER), progesterone receptor (PR), Human Epidermal Growth Factor Receptor type 2 (Her2) expressions and Ki 67 status were assessment, using immunohistochemistry (IHC) in 912 breast cancer patients. The IHC analysis is significant, yet it is almost impossible to pass in Uzbekistan. Cohort study included 2 groups of female BC: the 1 group of BC is Uzbek native women (500cases-54,8%), the 2 group of BC is women from another ethnics group (412-45, 2% cases).IHC assessments were grouped into five phenotypic subtypes: Luminal A (97cases), Luminal B Her2 negative (195cases), Luminal BC without Ki 67 score identification Her2 negative (246cases), Luminal B Her2 positive (79cases), triple negative (197cases) and non-luminal Her2 positive (98cases) (Fig. 5).
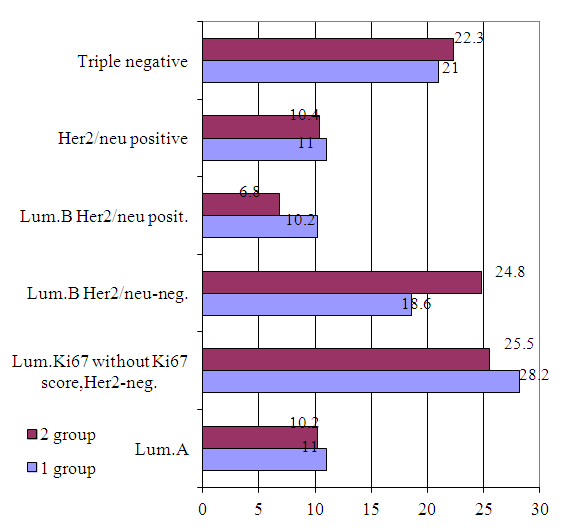 | Figure 5. Breast cancer molecular subtypes in two ethnic groups (n=912), % |
2.1. Results
- Most of Uzbekistan women with breast cancer are diagnosed relatively at young age and mostly have locally advanced stages and are treated with multimodality approach (Fig.8). Median age of BC in the 1 group is 48,5±11 years, 2 group is 52±11,4 years.Median age of Luminal A BC in the 1 group is 51,8±11 years, 2 group is 53,8±11,3 years. Median age of Luminal B Her2 negative BC in the 1 group is 49,6±12years, 2 group is 51,5±10years. Median age of Luminal B Her2 positive BC in the 1 group is 49,5±13,2years, 2 group is 49,5±13,5years. Median age of non-luminal Her2 positive BC in the 1 group is 46,6±7,4years, 2 group- is 53±8,3years. Median age of triple negative BC in the 1 group is 48,4±12 years and in the 2 group is 52,4±10,6 years. High nuclear grade (high nucleus-to-cytoplasmic ratio), high mitotic index and poorly differentiated all connote poor prognosis. Infiltrating ductal carcinoma is by far the most common type of invasive breast cancer, with relatively poorer survival. Tubular, medullary, mucinous, and papillary cancers have a more favorable prognosis, but account for only 6% of invasive cancers [18].The histological type of BC tumor was invasive carcinoma in 88,9% cases (Fig.6).
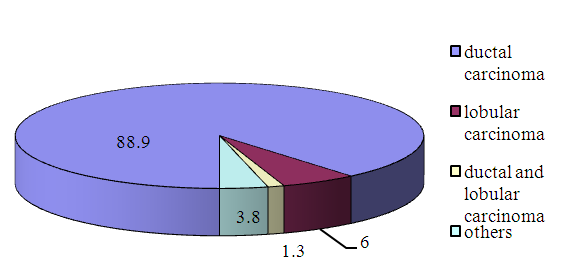 | Figure 6. The histological types of breast cancer (n=912) |
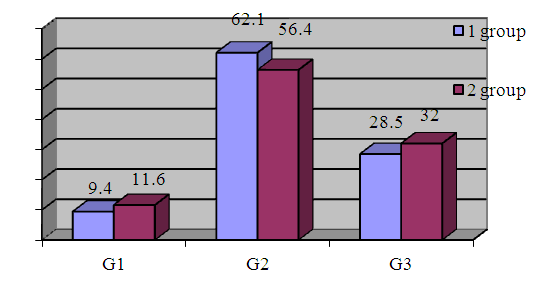 | Figure 7. Bloom-Richardson System With Nottingham Modification Scoring (n=912) |
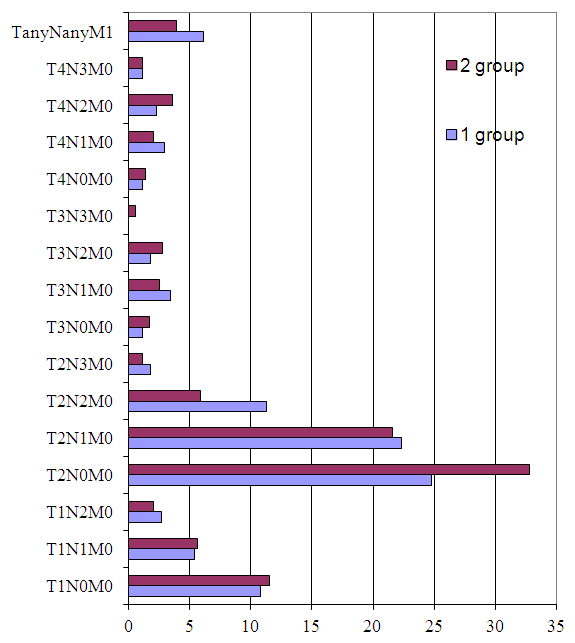 | Figure 8. Clinical characteristics of patients (n=912) |
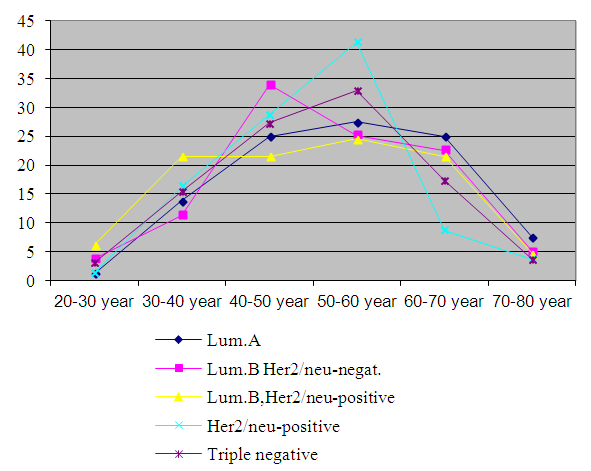 | Figure 9. Age and breast cancer subtypes in two groups of patients (n=666) |
|
2.2. Discussion
- In this cohort study from Uzbekistan, including more than 900 breast cancer patients, we found that subtypes of BC are heterogenic in ethnic groups. The breast cancer morbidity rate in Uzbekistan is constantly increased with the prevalence of locally advanced breast cancer. It is connected with no-effective working of national screening program.
3. Conclusions
- In this study, we first demonstrated heterogenic of breast cancer subtypes among Uzbekistan’s women. Our study findings suggest that the prevalence of triple negative (21,6%) breast cancer in Uzbekistan’s subjects is not similar to that among Western and Russian cohorts. Age is important risk factor for breast cancer, but it has also been suggested that age at diagnosis is related to breast cancer survival. Our findings hypothesize a positive association between Her2/neu-positive breast cancer tumors and hypothyroidism in breast cancer patients. These findings may have implications, and Her2/neu-positive breast cancer and thyroid’s function should be focused in larger breast cancer studies. In conclusion, there was a remarkable difference in tumor characteristics and biomarkers between Uzbek and other ethnic’s group cohorts. Uzbek women with breast cancer had a higher proportion of aggressive tumor types (triple negative and HER2-positive) than did other ethnic’s group. This study represents the first comprehensive assessment of the epidemiological features of breast cancer in Uzbekistan basing a framework for enhancing strategic health plans regarding breast cancer control in Uzbekistan.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML