-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2018; 8(6): 103-107
doi:10.5923/j.ajmms.20180806.01

Histopathologic Study and Prediction of Portosystemic Shunting Outcomes in Patients with Liver Cirrhosis
Nazirov Feruz Gafurovich, Baibekov Iskander Mukhamedovich, Babadjanov Azam Khasanovich, Salimov Umid Ravshanovich, Khakimov Dilshodbek Makhudovich, Ruziboev Sanjar Abdusalomovich, Ibadov Ravshan Alievich
Republican Specialized Scientific-Practical Medical Centre of Surgery named after Academician V.Vahidov, Tashkent, Uzbekistan
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Liver cirrhosis (LC) is one of major causes of global health burden. The Global Burden of Disease. There is no doubt that liver transplantation improves the survival rate and quality of life of end-stage LC patients. However, the list of eligible donors is always too short to help all those in need of liver transplantation. In addition, prediction of life expectancy is not accurate enough. Materioal and methods: Forty one liver biopsies taken from patients with cirrhosis who have lived from 1 year to 5 and over 10 years after portosystemic shunting were studied. The patients were distributed into the following three groups: group 1 (12 patients) who survived till 1 year, group 2 (14) survived till 5 years, and group 3 of 10 patients who survived 10 and more years. All biopsies were taken during surgical interventions. Guided fine needle biopsies were taken repeatedly from eight patients: under the control of spiral computer tomography and under the ultrasound control. Results: The histopathologic study was conducted in three groups. The most expressed changes in the liver parenchyma took place in the subcapsular region. Since liver cirrhosis results in a considerable thickening of the capsule, one should consider it when carrying out needle biopsies and their histopathologic study. The great part of unchanged hepatocytes was concentrated in the distal from the capsule areas. Conclusion: Efficiency of portosystemic shunts was determined by the number of affected hepatocytes, the severity of their necrobiotic changes and necrosis as well as by the ratio of morphologically unchanged hepatocytes to the affected ones. Liver cirrhosis, the capsule was considerably thickened. It should be taken into attention when carrying out puncture biopsies and their histopathologic study. The most expressed changes in the hepatic parenchyma took place in the subcapsular area.
Keywords: Liver cirrhosis, Portal hypertension, Histopathologic study, Portosystemic shunt
Cite this paper: Nazirov Feruz Gafurovich, Baibekov Iskander Mukhamedovich, Babadjanov Azam Khasanovich, Salimov Umid Ravshanovich, Khakimov Dilshodbek Makhudovich, Ruziboev Sanjar Abdusalomovich, Ibadov Ravshan Alievich, Histopathologic Study and Prediction of Portosystemic Shunting Outcomes in Patients with Liver Cirrhosis, American Journal of Medicine and Medical Sciences, Vol. 8 No. 6, 2018, pp. 103-107. doi: 10.5923/j.ajmms.20180806.01.
1. Introduction
- Liver cirrhosis (LC) is one of major causes of global health burden. The Global Burden of Disease 2010 study states that in 2010 it caused 31 million Disability Adjusted Life Years (DALYs), i.e. 1.2% of global DALYs, and one million deaths, or 2% of all deaths worldwide in that year [1]. In the 1990s, cirrhosis deaths began to increase in Central Asia countries. In 2010 the LC mortality made 54% (57% in 1990). According to the global statistics, in 2010 Kyrgyzstan had the fifth-highest cirrhosis mortality rate; while Uzbekistan and Turkmenistan ranked seventh and eighth, respectively [2].There is no doubt that liver transplantation improves the survival rate and quality of life of end-stage LC patients. However, the list of eligible donors is always too short to help all those in need of liver transplantation. In addition, prediction of life expectancy is not accurate enough [3]. In the past decade several predicting models were proposed, e.g. Child-Pugh score and MELD [4, 5]. Each of them has some advantages and disadvantages.Current hepatology assigns a special role to LC morphological study, its intraoperative biopsies above all. In the majority of liver diseases, this study is not only the critical factor of proper diagnosis; it also enables to determine the disease etiology. It helps to select the direction and tactics of further treatment, contributes to objective evaluation of its adequacy and efficiency and predicts the outcome of portosystemic shunting (PSS). Hence, intra-operative morphological study of the cirrhotic liver is expedient, and in some cases even mandatory. Wide introduction of transcutaneous needle biopsy of the liver, that has expanded opportunities of intravital morphological study, began in 1958 when G. Menghini published his article “One-second needle biopsy of the liver” in “Gastroenterology”. Nowadays liver biopsies and their histopathologic study remain the procedure of choice both for making diagnosis (and its verification in particular) and monitoring the pathomorphism of almost all liver diseases and various methods of their treatment [12].Compensatory adaptation of the intact liver parenchyma is known to level the limited pathological changes of the organ. The reserve capacities of the liver are so great that even a damage to a great number of hepatocytes can result in no clinical and biochemical manifestations. The clinical symptoms can appear only when the total number of damaged cells becomes critical. Thus, the morphological study quite often is an effective method of early clinical diagnosis [12].Intra-operative biopsies, enabling to obtain specimens of optimal sizes and rationally choose the target-place, are at least not less important than the ones taken after the surgery. According to the generally accepted rule, a surgeon should cut off some liver tissue for morphological study immediately after opening the abdominal cavity. Biopsies taken in the end of surgical procedure can lead to the picture of retractor-associated inflammatory infiltration and subcapsular impairment of the liver parenchyma caused by surgical injury to the tissue. It is necessary to emphasize that the subcapsular area cannot represent a picture of the entire organ. Expressed fibrosis in this area can co-exist with the minimum impairment and changes in the deeper parenchyma [6-8].Currently cirrhosis is becoming an increasingly common diffuse liver impairment. Despite the significant advances in diagnosis and treatment of liver cirrhosis (LC), including surgical methods, the disease is one of the few with steadily rising death rate. However, liver cirrhosis can develop without characteristic clinical manifestations and may come to light only by post mortem examination. Developed LC is known to be a non-stationary pathology: it can progress. The dynamics of these changes depends on fibroblast activity in septa and small nodules, the presence of hepatocyte necrosis, the condition and features of the liver blood supply, etc. Objectively, only biopsies enable to determine cirrhosis severity and profile [6, 8].Despite few, morphologically proven cases of cirrhotic liver restoration [9], liver cirrhosis is considered irreversible. Therefore, the attention of current surgery to cirrhosis is caused not by its possible reduction, but rather by efficiency of correction of portal hypertension and its complications using various portosystemic shunts (PSS). However, by now, no definitive algorithm of morphological evaluation of the liver has been developed to determine PSS expediency.The available literature on liver morphology in PSS concerns mostly with the morphological criteria of LC activity and fibrosis degree as well as the impact of various types of porto-caval shunts on these parameters [10, 11]. In addition, morphologists interpret differently the expressiveness of structural changes in vessels bringing blood to the liver, and sinusoids as well as veins bringing blood from the cirrhotic liver. For instance, R.H. Kelty et al. [11] revealed that in cirrhotic transformations of the liver, obstruction occurred in sinusoids near the central veins with the central veins displaced and spread around regenerating pseudo-lobules while the portal veins were much less affected.The histopathologic study of intra-operative biopsies, taken from 13 LC patients 6-15 months after PSS [12], initially demonstrated cirrhosis with the expressed impairment of the lobule structure, cyto- and angio-architectonics of the organ in 84.6% of cases under study. In other cases, the lobule structure was intact, and cirrhosis with active regeneration of the parenchyma and formation of false lobule was developing. In 92.3% of cases, the central veins were significantly deformed up to their poor visualization. The researchers believe that expressed dilatation of the venules included in the portal triads in developed cirrhosis can be interpreted as an extremely unfavorable factor predicting the PSS outcome since in early postoperative period the risk of development of retrograde portal blood flow increases. However, interpretation of the same sign, e.g., in the initial stages of LC development, when the lobule structure is not impaired or in Budd-Chiari syndrome, can be a little different because, in these cases, the bed of microvesseles taking blood from the liver is much less affected. Therefore, to elaborate the criteria of morphological evaluation of the liver and determine interrelation between the liver structure during surgeries and the duration of survival we have analyzed 41 liver biopsies taken from LC patients operated in the NSPCS from 1979 to 2015. The follow-up period has lasted for 13 years.
2. Material and Methods
- The patients were distributed into the following three groups: group 1 (12 patients) who survived till 1 year, group 2 (14) survived till 5 years, and group 3 of 10 patients who survived 10 and more years. All biopsies were taken during surgical interventions. Guided fine needle biopsies were taken repeatedly from eight patients: under the control of spiral computer tomography “Somatom Balance” (Siemens, Germany) (5 cases) and under the ultrasound control with “Aloka SSD-630” (Japan) (3 cases) at various periods after surgery (from 3 patients after 3 years, from other 3 ones after 5 years and 2 after 10 years since PSS).The tissue specimens for light microscopy were fixed in 12% formalin solution on the phosphate buffer by Lillie’s technique. Paraffin sections were stained by hematoxylin - eosin. The light-optical microphotos were taken with “Axioscope” (Zeizz) microscope with "Sony" digital chamber and subjected to computer processing on computer Computek Pentium IV with Windows XP software.
3. Results and Discussion
- Our histological study demonstrated that the greatest structural changes in the liver developed in group 1 consisting of the patients died within the first year after PSS. It revealed that mixed multilobular cirrhosis dominated in this group. Small monolobular nodules alternated with domination of the large ones. Wide fields of coarse-fiber connecting tissue were located between them. The cells forming the small nodules were of different size and form. The hepatocyte cytoplasm, as a rule, was light. The nuclei were also polymorphic. However, the cells with medium-sized nuclei dominated. The cells often had two and even three nuclei; some cells were in the condition of necrobiosis and necrosis (Figure 1). In small nodules, the central veins were not detected.In the majority of cases under study, the liver capsule was thickened. Under the capsule, the extensive areas of hemorrhages and expressed lymphoid infiltration were quite often identified. Aggregations of dilated lymphatic capillaries were often located under the capsule. Bridging and piecemeal necroses were frequent; quite often they were combined with each other in one area. Bridging necrosis made the border between septa and small nodules vague. The presence of both piecemeal and bridging necrosis (Figure 1) is an unfavorable predicting sign. Piecemeal necrosis was accompanied by expressed round cell lymphoid infiltration. In this group of patients, the expressed dilatation of sinusoid capillaries was another structural characteristic of the liver in the areas where the lobule architectonics was more or less preserved (Figure 2). The areas of the liver with normal architectonics, as a rule, were found in deeper parts of the organ and were usually accessible to study only through intra-operative biopsies when it was possible to obtain large enough amount of the tissue.
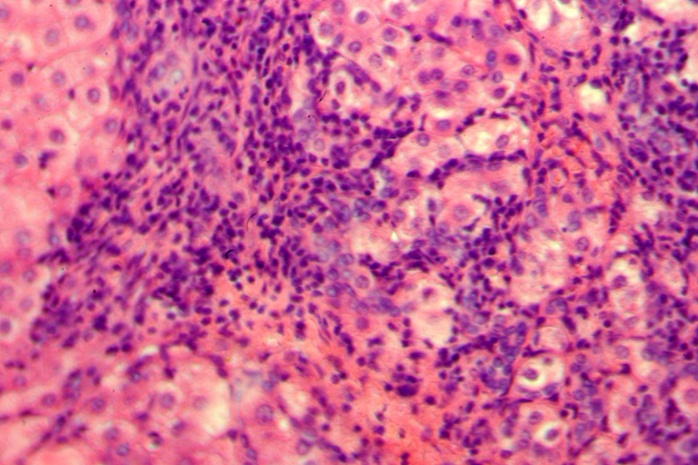 | Figure 1. Group 1: piecemeal necrosis, lymphoid infiltration, necrobiosis and polymorphism of hepatocytes and their nuclei. Hematoxylin-eozin, H-E, 10х20 |
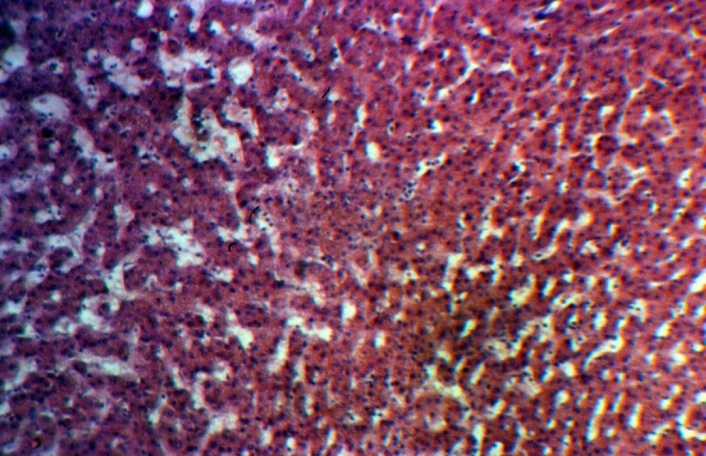 | Figure 2. Group 1: the dilatation of sinusoids in deep parts of the liver, H-E, 10х10 |
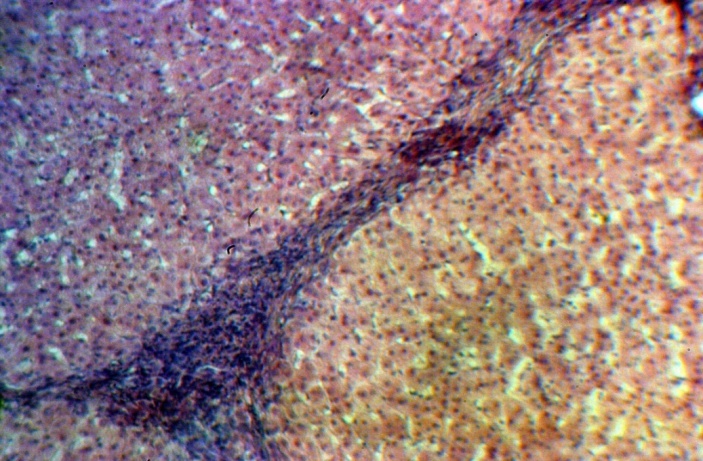 | Figure 3. Group 2: rather narrow septa; satisfactory condition of the hepatocytes is, H-E, 10х10 |
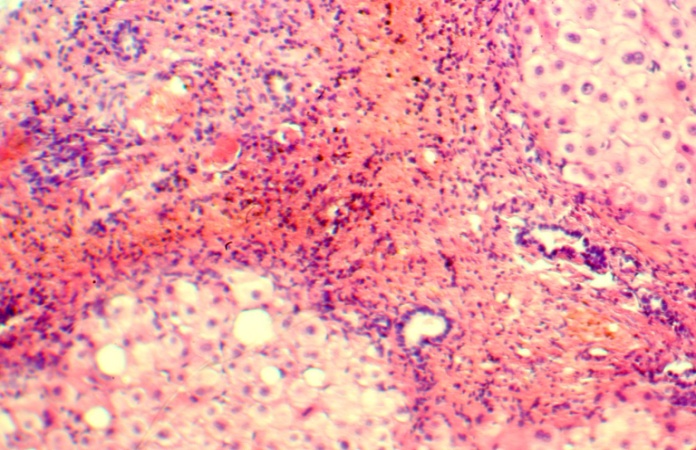 | Figure 4. Group 2: bile ducts and septa vessels, H-E 10х20 |
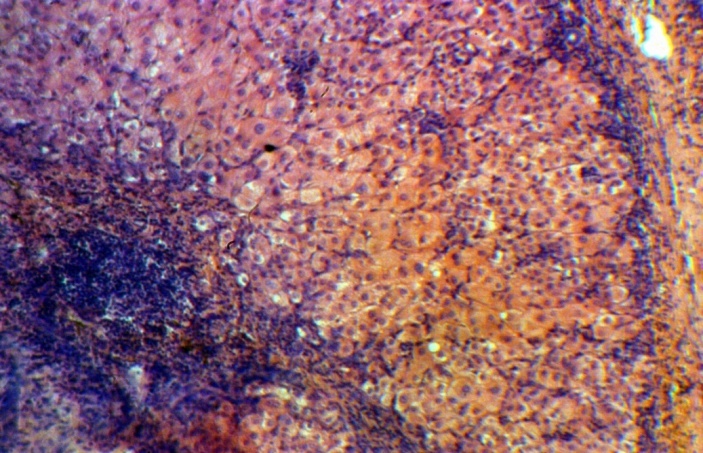 | Figure 5. Group 3: the hepatocytes with marked infiltration of septa and lobules, H-E 10х20 |
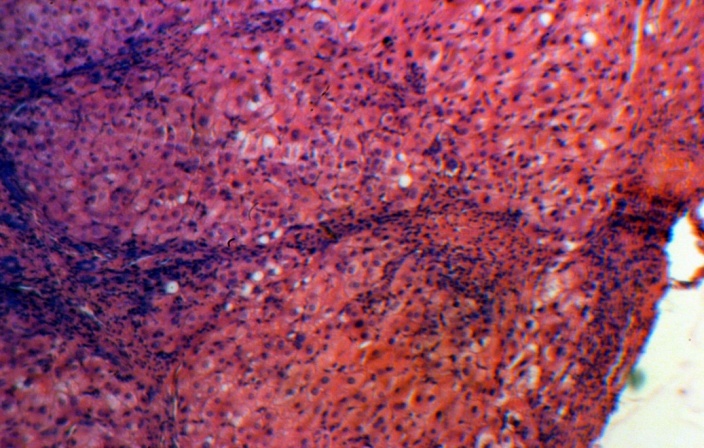 | Figure 6. Group 3: the narrow septa, satisfactorily preserved hepatocytes, H-E 10х10 |
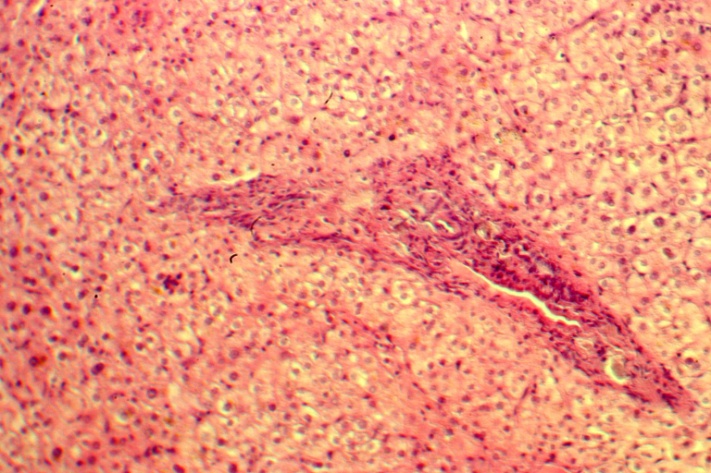 | Figure 7. Well preserved hepatocytes; small growth of connective tissue around the portal tract. Five years after PSS. H-E 10х10 |
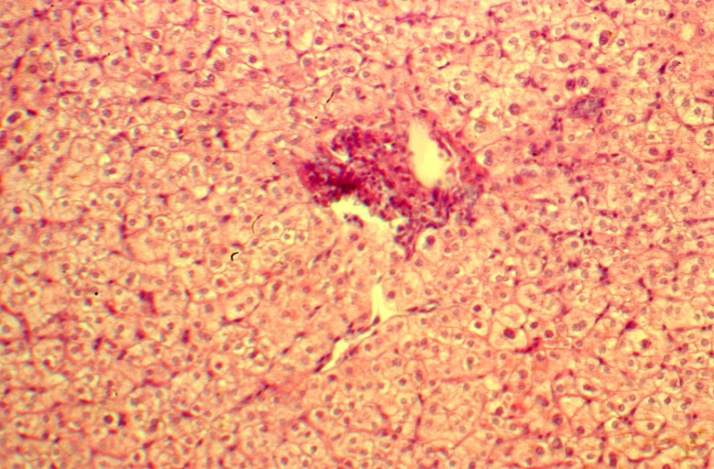 | Figure 8. The liver architectonics; small growth of connective tissue around the portal tract; 13 years after PSS. H-E 10х10 |
4. Conclusions
- In liver cirrhosis, the capsule was considerably thickened. It should be taken into attention when carrying out puncture biopsies and their histopathologic study.The most expressed changes in the hepatic parenchyma took place in the subcapsular area. However, prediction of PSS outcomes should be done using the distal from the capsule areas of the liver parenchyma. In other words, it should be done using the ratio of unchanged hepatocytes to changed ones keeping in mind that the presence of bridging and piecemeal necroses suggests the unfavorable prognosis. Special information could be obtained by morphological research of microcirculation bed impairment in the cirrhotic liver with detailed studying the degree of mutual relations of blocks to portal blood circulation at the pre- and post-sinusoidal levels.Ethical approval. The review board and ethics committee of Republican Specialized Center of Surgery named after acad. V. Vakhidov. Tashkent. Uzbekistan approved the study protocol and gave permission.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML