-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2018; 8(4): 79-90
doi:10.5923/j.ajmms.20180804.05

Urinary Metabolomic Profiles and Netrin-1 as Diagnostics and Predictors of Acute Kidney Injury in Preterm Neonates
Eman A. Al Morsy1, Entsar R. Mokhtar2, Gamil E. Ibrahim3, Asmaa M. El- Nasser4, Eman E. Ebrahem5, Shahinaz Elattar5
1Pediatrics and Neonatology Department, Faculty of Medicine (for Girls), Al-Azhar University, Cairo, Egypt
2Clinical Pathology Department Faculty of Medicine (for Girls), Al-Azhar University Cairo, Egypt
3Chemistry of Flavor & Aroma Department, National Research Center, Dokki, Egypt
4Department of Medical Microbiology and Immunology, Faculty of Medicine (for Girls), Al-Azhar University, Cairo, Egypt
5Department of Medical Biochemistry, Faculty of Medicine (for Girls), Al-Azhar University, Cairo, Egypt
Correspondence to: Entsar R. Mokhtar, Clinical Pathology Department Faculty of Medicine (for Girls), Al-Azhar University Cairo, Egypt.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background: Acute kidney injury (AKI) has a higher incidence in preterm neonates, because full nephrogenesis is not completed until week 36 of gestation. Delay in the diagnosis of AKI using conventional biomarkers has become one of the important obstacles in applying early effective interventions. Handling of major biochemical classes of metabolites occurs in the kidneys. So, any changes in urinary metabolite concentrations may reflect impaired kidney function. Urinary Netrin-1(NTn-1) is a promising early up- regulated diagnostic marker of AKI across many types of injury. Objective: To asses metabolomics data obtained from the urine of preterm infants to uncover biomarkers that may be used in diagnosing or predicting the development of AKI, and to investigate the values of urinary netrin-1 in the early diagnosis of AKI. Methods: This study was conducted on 40 preterm neonates, they were admitted to the neonatal intensive care unit (NICU) of Al-zahraa university hospital. They were sub-classified into 2 groups: 20 neonates with AKI and 20 neonates with no- AKI. Urinary metabolomic profiles (UMP) of these neonates were determined by using high performance liquid chromatography (HPLC) and, urinary NTn-1 was measured by ELISA. Results: Metabolomics analysis revealed significant changes in 7 urine metabolites. They were: homovanillic acid, carnosine, betaine, glucose, allantoin and acetic acid which were upregulated, while hippuric acid was downregulated. Urinary Netrin-1 levels were significantly higher in AKI neonates as compared with no-AKI ones (p =0.000), and its level was positively correlated with urea and serum creatinine (sCr) (r = 0.520, p = 0.019; r = 0.482, p = 0.032) respectively. Conclusions: Urinary metabolomics is a promising tool to identify markers of AKI. They can identify at risk neonates before the disease process goes on, which may be helpful in early diagnosis and treatment before irreversible structural injuries occur. Netrin-1 is an early up- regulated biomarker for detection of AKI.
Keywords: Acute kidney injury, Preterm neonates, Metabolomic profiles, Netrin-1
Cite this paper: Eman A. Al Morsy, Entsar R. Mokhtar, Gamil E. Ibrahim, Asmaa M. El- Nasser, Eman E. Ebrahem, Shahinaz Elattar, Urinary Metabolomic Profiles and Netrin-1 as Diagnostics and Predictors of Acute Kidney Injury in Preterm Neonates, American Journal of Medicine and Medical Sciences, Vol. 8 No. 4, 2018, pp. 79-90. doi: 10.5923/j.ajmms.20180804.05.
Article Outline
1. Introduction
- With advancement in neonatal care of extremely low birth weight (ELBW) and preterm infants, there was large improvement in the survival of this critically ill neonates, this improvement in survival comes at the cost of short-term and long-term sequels [1], [2]. There was a significant advancements in understanding AKI and its effect on outcomes across neonates [3]. Acute kidney injury is common in premature infants in NICU and predicts poor clinical outcome [4]. Clinically AKI is defined as a rapid decrease in kidney function which result in failure to maintain electrolyte, fluid, and acid-base homoeostasis [5]. The most common form of AKI in hospitalized neonates is acute tubular necrosis (ATN) [6], which is caused by ischemic or toxic injury to the tubular epithelial cells resulting in tubular dysfunction [7]. In premature infants, the tubular function is immature, with a decreased ability to reabsorb protein and electrolytes and to concentrate urine. Immaturity worsens the natural neonatal kidney vulnerability to ischemic and hypoxic insults, mainly caused by higher perfusion rate, and vulnerability to potentially exogenous or endogenous toxic substances that may be present in the circulation [8]. Premature infants are twice as likely to develop chronic kidney disease (CKD) and kidney failure compared with term infants [4]. Children who survive AKI as infants are often more susceptible to CKD later in life [9]. Premature infants may be born with less than half of the nephrons compared with term neonates, predisposing them to CKD early in life and as they age. AKI in neonates is often multifactorial and may result from prenatal, perinatal, or postnatal insults as well as any combination thereof [10]. These neonates are exposed to a variety of external stressors that can interfere ongoing kidney development or cause additional nephron loss such as, nephrotoxic medications, infections, hemodynamic alterations and suboptimal nutrition [2]. Traditional markers of renal function, including sCr and urea are not specific and only increase significantly after substantial kidney injury. Serum creatinine is a measure of function not injury, and it is a late marker for an acute injury as more than 50% of nephrons must be compromised before changes in the sCr level become evident. Furthermore, at lower glomerular filtration rate (GFR), sCr overestimate renal function, due to tubular secretion of creatinine; sCr varies with age, gender, muscle mass, hydration status, and finally, bilirubin and medications can affect sCr measurement [11]. Also renal tubule damage, as found in cases of ATN, can be insufficient enough to cause a change in currently used parameters of kidney function such as sCr or urea levels, thus complicating their use as potential markers of AKI in preterm neonates [12]. Therefore, more sensitive markers of kidney injury are needed. The ideal biomarkers will identify AKI early in the disease process, which help preventive strategies and maximize the potential for effective interventions [5]. Metabolomics is the measurement of metabolome in biological matrices, which provides a systematic view of the change of all metabolites in the physiological or pathological conditions [13]. It is the process which describes the phenotype of a cell, tissue or organism through the full complement of metabolites present. They measure global sets of low molecular weight metabolites (including sugars, organic acids, amino acids, lipids, fatty acids, steroids, small peptides, vitamins, etc.), thus it captures a “snapshot” of the metabolic status of a cell, tissue or organism in relation to external stimuli or genetic variations at a particular time [14]. Many of these markers have been confirmed in previous studies as, they can detect nephrotoxicity earlier than histopathology and the traditional clinical chemistry methods. Thus the use of metabolomics appears to be a promising tool in neonatal nephrology [15]. Moreover, the change of specific metabolites may indicate the pathways that are critically important in the pathogenesis of AKI and subsequent recovery. In addition, the change of certain metabolites in the early phase of AKI may suggest their possibility of serving as biomarkers for the diagnosis of AKI [13]. AKI is a metabolic disease that results from hypoxia and subsequent necrosis and/or apoptosis of renal tubular epithelial cells, so metabolomics analysis of the urine will result in biomarkers, that reflect the altered metabolic function of these cells [16]. In neonatal and pediatric nephrology, urine is considered the ideal non invasive sample, which can be collected easily (a spot sample is adequate), since it is a so-called “proximal matrix,” being closer to (or in direct contact) with the kidney, which is the site of disease [17]. Consequently, the urine metabolome reflects kidney pathophysiological changes, while metabolome in whole blood, serum and plasma reflects systemic changes. Also, the urine metabolome includes the intermediate metabolites, which reflects specific metabolic processes [18].Among other biomarkers of AKI, Netrin-1 which belongs to the laminin-related family [19], it plays an important role in the formation of new blood vessels and cell adhesion [20]. It is highly expressed in many organs including kidney [21]. Within the kidneys, netrins are normally located in the peritubular capillaries of the kidneys, but during ischemia reperfusion injury (IRI), Ntn-1 is highly induced within the tubular epithelial cells [22]. Netrin-1 regulates inflammation and inflammatory cell migration during AKI through the suppression of cyclooxygenase (COX-2) induction of prostaglandin (PGE2) and thromboxane A2 production [23]. NTn-1 provides some protection from IRI, potentially by the suppression of oxidation, inhibition of neuropeptide Y expression [24], and by promoting kidney epithelial cell proliferation and inhibiting apoptosis [25]. Compared with other biomarkers of AKI as kidney injury molecule-1 (KIM-1), neutrophil gelatinase associated lipocalin (NGAL), cystein rich protein-61(Cyr61) and N-acetyl-b-D glucosaminidase (NAG), NTn-1 is secreted into the urine rapidly when kidney damage occurs and is returned to the normal level after perfusion; thus, NTn-1 can also be used to evaluate the prognosis of renal function recovery [26].Early identification of newborns with AKI represents the first step in the prevention of this condition. Appropriate treatment of AKI, improves the outcome and prognosis. It reduces the morbidity and mortality of AKI in critically ill newborns and also reduces hospital stay and medical costs [9]. So, our purpose of this study was to use metabolomics data obtained from the urine of pre-term infants to uncover biomarkers that may be validated in future studies for use in diagnosing or predicting the development of AKI, and to determine whether changes in the urinary excretion of NTn-1 can be used as an early biomarker of AKI.
2. Patients and Methods
2.1. Study Design
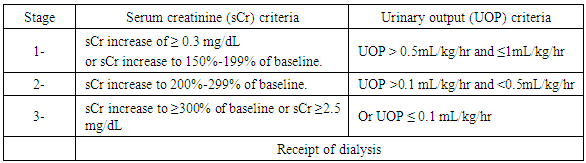
- This comparative cross sectional study was conducted on 40 preterm neonates, with respiratory distress syndrome (RDS), their gestational age ranged from 28 to 33 weeks. They were sub-classified into 2 groups according to development of AKI: 20 neonates with AKI and 20 neonates with no AKI. Twenty neonates who met the diagnostic criteria for AKI as in table (1) were selected as cases. All AKI cases had lower Apgar score and lower gestational age than no-AKI ones and they were categorized in stage 1 and 2.Baseline sCr will be defined as the lowest previous sCr value [27]. Neonates considered for this study were selected from NICU of Al-zahraa University Hospital, during the period from November 2016 to December 2017. Full term neonates, or premature one with: neonatal sepsis, severe asphyxia, multiple congenital anomalies including renal or any other congenital anomalies, newborn experienced exchange transfusion, extreme low birth weight, inborn error of metabolism, and also newborn to the mother who suffered from CKD were excluded from this study. An Informed written consent was obtained from parents of these neonates in adherence with the guidelines of the local ethical committee. All the studied neonates were subjected to, Full history taking, including prenatal history, maternal condition during pregnancy (diabetes, hypertension and pre mature rupture of membrane). Natal history includes duration and mode of delivery. Post-natal history including 1st cry, cyanosis, Apgar score at one minute 5, and 10 minutes, medications, sleepiness, respiratory distress and convulsions. Clinical examination including, assessment of gestational age using New Ballard Score [28], Score of neonatal acute physiology (SNAP-II SCORE) for prediction of mortality and morbidity [29]. Anthropometric measures, oxygen support, length of ventilation, length of hospital stay and outcome. All cases were managed according to nursery protocol.
|
2.2. Blood and Urine Sampling and Measurements
- a) Blood sample collection: 2 ml of venous blood was collected into serum separator tube, left to clot then centrifuged at 3500 rpm for 10 min, then serum was separated and sent to chemistry lab for urea and sCr measurements using COBAS C311 auto analyzer (Roche Diagnostics GmbH, Mannheim, Germany), serum Na and K measurements using 9180 Electrolyte analyzer (Roche Diagnostics GmbH, Mannheim, Germany). Another 0.5ml of blood was taken on heparinized syringe for blood gases determination, using Rapid Lab 348 (Siemens. Health care Diagnostics, Inc, UK).b) Urine sample collection: urine samples were collected aseptically from all neonates after 72 hrs of delivery to overcome false urinary levels reflecting those of maternal kidney function due to placental transfer [30]. It was collected by using a sterile plastic bag because bacteria metabolism significantly interferes on the urine metabolome [18]. This plastic bag has a sticky strip on one end, which made to fit over baby’s genital area. The plastic bag was opened and placed on the infant; for males, the entire penis was placed in the bag and the adhesive strip was attached to the skin. For females, the bag was placed over the two folds of skin and attached on either side of the labia. A diaper was put on the baby (over the bag), the infant was checked often, and the bag was changed after the infant has urinated, then urine output was collected. The urine was evacuated from the bag into three tubes, first tube with about half ml of urine was used for PH determination using Indicator paper Uralyt-U (Lot Number: 32085900). The second tube with another 1ml of urine was centrifuged at 15,000 rpm for 10 min to remove any particulates, then the supernatant was collected and stored at -20°C for further metabolomics analysis. We did not add any preservatives to the urine sample because urinary metabolites are stable for about 4 weeks [31], and when samples were stored frozen, the use of preservatives has little to no effect on the resulting metabolomics profiles [32]. The third tube with 1 ml of urine was centrifuged for 20 min at 25000 rpm, then the supernatant was collected and stored at -20°C for further urinary Netrin-1 analysis. It's measurement was done by using commercially available human Netrin-1 ELISA kits, which was supplied by Elabscience Biotechnology Inc, catalog number: E-EL-H2328. Urinary NTn-1 level measurement was done on ELISA system (reader; A1851 Das, Italy, and washer; 16041412 Bio Tek, USA), according to manufacturer's instructions.
2.3. HPLC-MS Metabolomics Analysis
- Sample PreparationThe urine samples of all neonates were thawed before analysis and centrifuged for 5 min at 12000 xg. A 50 μL aliquot of the supernatant of each sample was diluted with 50 μL of Milli-Q water and vortex mixed; the resulting solution was transferred to HPLC for metabolomics analysis.HPLC-MS procedureMetabolomics analysis were done on the HPLC Agilent 1100 series (Waldborn, Germany), quaternary pump (G1311A), Degasser (G1322A), Thermostated Autosamples (G1329A), variable wave length detector (G1314A); and column: Zorbax 300SB C-18 column (250 mmX4.5mmX 5um) (Agilent Technologies, USA). The solvent system consisted of methanol with 0.1% formic acid (solvent A); acetonitrile with 0.1% of formic acid (solvent B). The gradient system was programmed as follows: starting at 30% solvent B, increasing to 60% over 10 min, increasing to 100% over 5 min, and then returning to 30% over 5 min. The injection carried out under ambient temperature. Mass spectra were acquired in positive ion mode in a mass range of m/z 100–800 amu at a fragmentor voltage of 70 eV. Nitrogen was used as drying gas at a flow rate of 10 ml/min. Nebulizer pressure was set to 45 psi. For all spectra manual baseline subtraction was performed. The concentration of each metabolite was normalized to each urine sample’s corresponding creatinine value to compensate for variations in urine volume (the concentration of metabolites is expressed as uM/mM creatinine.
2.4. Statistical Analysis
- Data was Statistically analyzed by Statistical Package for Social Science (IBM SPSS) version 23. The quantitative data were presented as mean, standard deviations and ranges when their distribution found parametric and median with inter-quartile range (IQR) when their distribution found non-parametric. Also qualitative variables were presented as number and percentages. The comparison between groups regarding qualitative data was done by using Chi-square test. The comparison between two independent groups with quantitative data and parametric distribution were done by using Independent t-test while non parametric data were compared using Mann-Whitney test. Spearman correlation coefficients were used to assess the correlation between two quantitative parameters in the same group. Logistic regression analysis was used to assess the predictors of AKI with odds ratio and 95% confidence interval (CI). Also Receiver operating characteristic curve (ROC) were used to assess the best cut off point with sensitivity, specificity, positive and negative predictive value and area under curve (AUC) for the predictors of AKI patients. The confidence interval was set to 95% and the margin of error accepted was set to 5%. So, the p-value was considered significant at the level of < 0.05.
3. Result
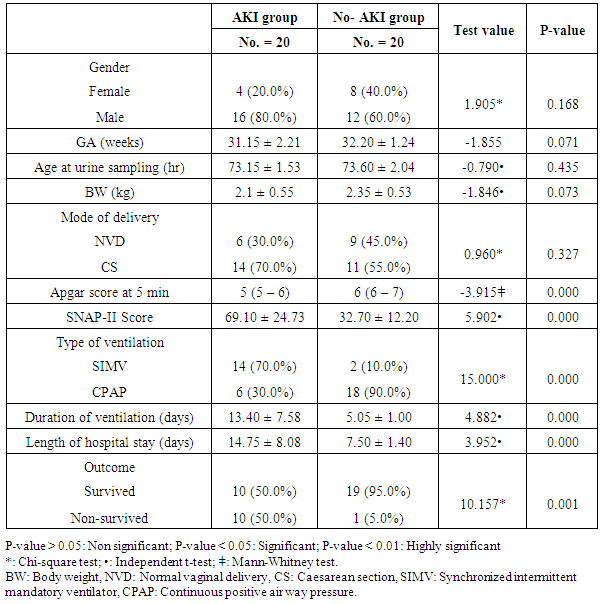
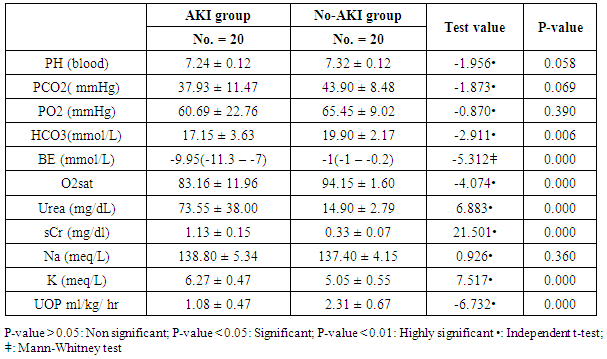
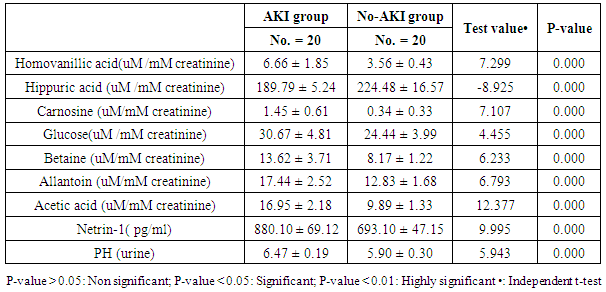
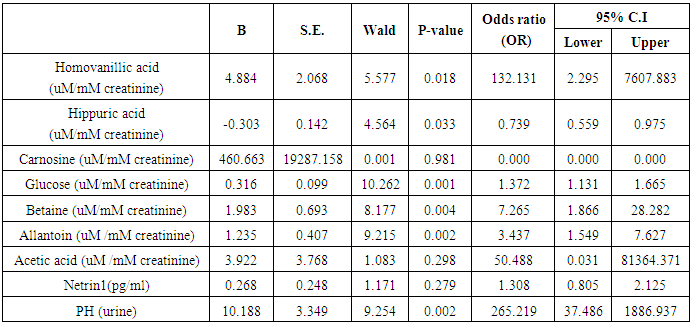
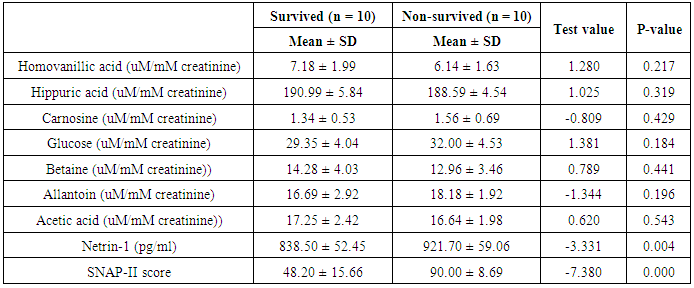
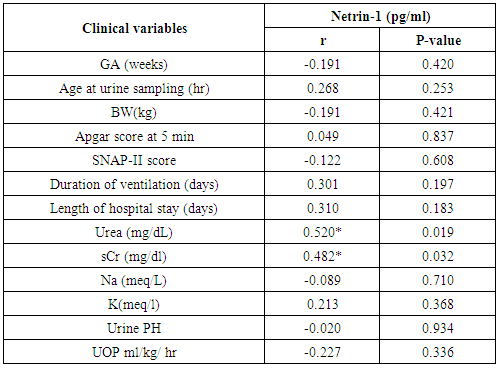
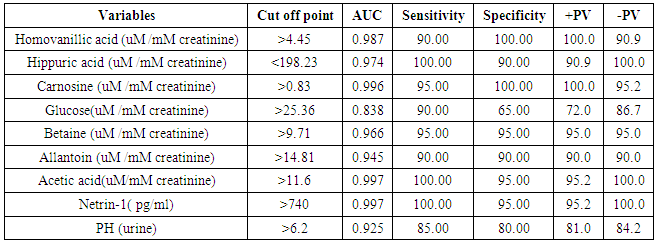
- The results of this study showed statistical significant differences between AKI and no-AKI neonates in terms of Apgar score at 5 minutes, SNAP-II score, type of ventilation, duration of ventilation, length of hospital stay and outcome (Table 2). Also, there was statistical significant difference between the two studied groups regarding HCO3, BE, O2sat, urea, sCr, serum potasium and UOP (Table 3). There was highly significant increase in the levels of homovanillic acid, carnosine, glucose, betaine, allantoin and acetic acid in the urine of AKI neonates as compared with no-AKI ones (p = 0.000) for all metabolites. While, hippuric acid level was significantly lower in urine from AKI neonates as compared with no-AKI ones (p = 0.000). Urinary Netrin-1 levels were significantly higher in AKI neonates as compared with no-AKI ones (p = 0.000). Also, there was significant difference in urine PH between AKI and no- AKI neonates (Table 4). Univariate logistic regression analysis for predictors of AKI showed that; urinary levels of homovanillic acid, hippuric acid, glucose, betaine, allantoin and PH were associated with AKI, while urinary levels of carnosine, acetic acid and NTn-1 were not associated with AKI, as shown in (Table 5). Comparison between survived and non-survived neonates in AKI group showed higher levels of urinary Netrin-1and SNAP- II score in non survived neonates than those who survived (Table 6). Urinary Netrin-1 levels were positively correlated with urea and sCr (r = 0.520, p = 0.019; r = 0.482, p = 0.032) respectively (Table 7), (Figure 1, 2). Roc curve analysis of urinary metabolomic profile, urinary Netrin-1 and PH were illustrated in (Table 8), (Figure 3, 4).
|
|
|
|
|
|
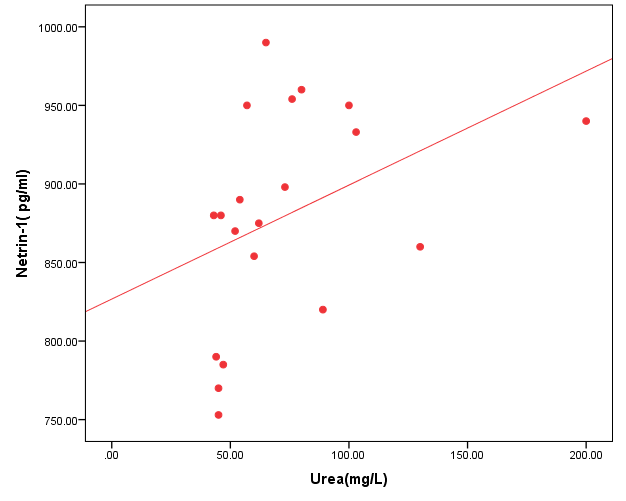 | Figure (1). Correlation between urinary Netrin-1 level and urea |
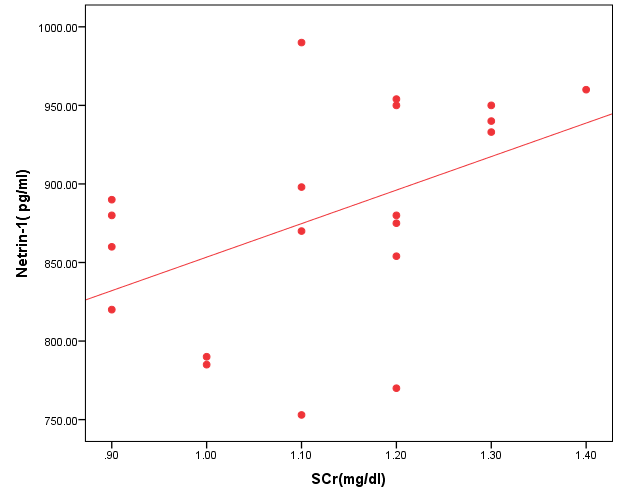 | Figure (2). Correlation between urinary Netrin-1 level and serum creatinine |
|
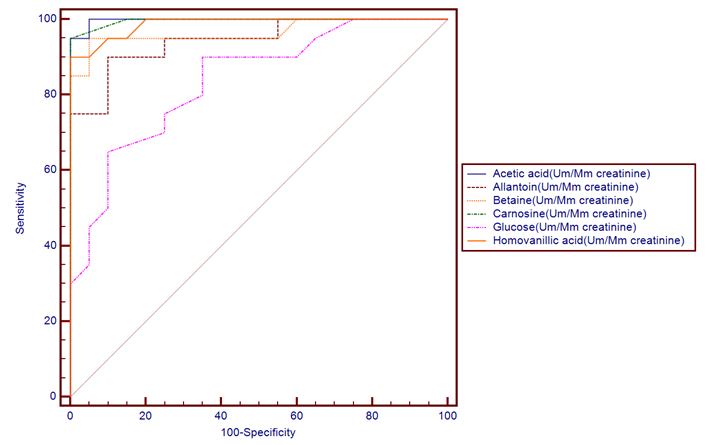 | Figure (3). ROC plot for markers of metabolomic profile |
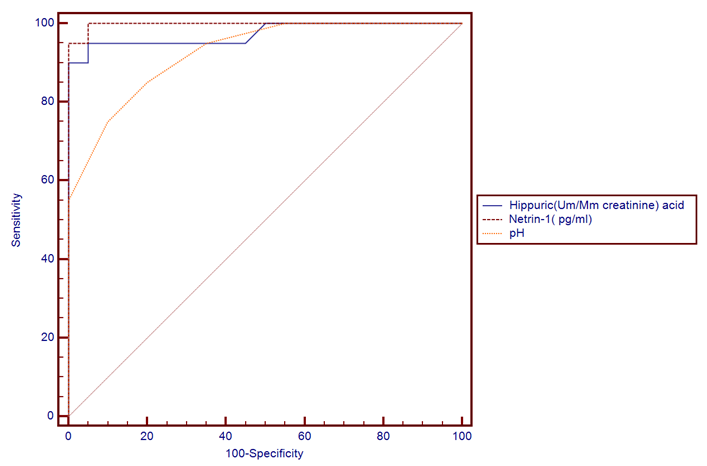 | Figure (4). ROC plot for markers of metabolomic profile, Netrin-1 and PH |
4. Discussion
- The incidence of neonatal AKI is the highest one followed by adults and children [9]. Biomarkers used currently for AKI, as sCr, urea and NGAL, are insensitive enough for timely detection of AKI in preterm infants [33]. Failure of early diagnosis and treatment of AKI is due to lack of sensitive and specific renal markers which help in early detection of impending AKI [34]. Ideal biomarkers of AKI would be up-regulated soon after renal injury and would be independent of GFR. In premature infants, extra-uterine renal development impacts the ability to accurately monitor changes in potential AKI biomarkers, which take place over the first few days or weeks of life creating a further challenge in the discovery of biomarkers of AKI in this population [11]. Potential biomarkers of AKI might show up in urine during tubular inflammation or tubular dysfunction, leading to proteins, enzymes, or small molecule metabolites in urine that are generally filtered at the glomerulus [35]. In this study, we analyzed the urine samples from 40 neonates with the goal of identifying a metabolic biomarker of AKI. Our results showed that the metabolic profile of neonates with AKI was markedly different compared to those with no-AKI. Metabolomics analysis revealed significant changes in 7 urine metabolites, which indicate altered metabolites in catecholamine, benzoate, protein, carbohydrates and purine metabolism. Our study showed that metabolic profile changes in urine are a surrogate molecular marker sensitive enough to detect the pathogenetic changes of AKI. In this study we found higher levels of homovanillic acid (a major catecholamine metabolite) in AKI neonates when compared with the no-AKI ones, this was consistent with previous study done by Beger et al. [36] who applied a metabolomic analysis of urine to the study of AKI as a complication following cardiopulmonary surgery in the pediatric age. They identified a metabolite of dopamine, homovanillic acid, to be a reliable maker of AKI with 90% sensitivity and 95% specificity using a cutoff value of 24ng/ml at 12 h after surgery. While we found homovanillic acid at a cutoff point >4.45uM/mM creatinine, the sensitivity was 90% and the specificity was100%. The increase in homovanillic acid in this ischemic-reperfusion model of AKI may be due to disturbed homeostasis of the catecholamine system in the kidney.On the other side Mercier et al. [37] showed that, lower homovanillate can differentiate AKI from no AKI neonates, and homovanillate has been previously implicated in nephrotoxicity [38]. They demonstrated that urinary metabolites can differentiate between neonates with and without AKI. Hippuric acid (a major xenobiotic metabolite) is a glycine conjugate of benzoic acid, it is related to diet and the gut microbiome, that is secreted by the proximal tubule, and it has been implicated as a marker of the health of the proximal tubule. Increased urine hippuric acid content may have antibacterial effects [39], [40].We found lower levels of hippuric acid in AKI neonates as compared with no-AKI ones, which may be due to induction of aminoacylase enzyme that metabolize hippurate to benzoate [41]. Other possibility is that in AKI, the early decline in blood flow in the kidney and decreased renal function is expected to decrease urinary excretion of hippuric acid by the proximal tubule which indicative of a malfunction of the kidney tubule [40]. Our result was matched with Mercier et al. [37] who reported that, lower hippurate can differentiate AKI from no AKI profiles, reflecting alterations in uremic toxin elimination. Our result also was in agreement with Lorenzo et al. [42] who reported that urinary hippuric acid was significantly downregulated in AKI patients. They demonstrated that hippuric acid was associated with the severity of AKI and its level was lower in ATN than in the prerenal condition. Early kidney damage was found to be associated with a decrease of hippuric acid in urine, also, reduced urinary hippuric acid has been found to be an early event in endogenous nephrotoxin or drug-induced tubular injury [43]. Hippuric acid was one of the metabolites showing variation in urine following administration of a single dose of the nephrotoxic agent cyclosporine to healthy volunteers [41]. Carnosine is an endogenous dipeptide of the amino acids histidine and beta-alanine. It plays a role in tubular and glomerular function. It has an ability to scavenge reactive oxygen species with antioxidant activity, it has a buffering effect which help to maintain the acid base balance [44]. Carnosine reduce the synthesis of matrix proteins such as collagen type VI and fibronectin in messangial cells and podocytes, and acts as a natural angiotensin converting enzyme inhibitor [45]. In the present study carnosine was found to be increased in urine samples from AKI neonates as compared with no-AKI ones, suggesting that these patients were having greater renal dysfunction and that urinary carnosine was increased in these infants due to failed or reduced proximal tubular reabsorption, which is a common symptom of ATN, and it is known to be the final step leading to onset of AKI. This could be directly attributed to a dysfunction in the epithelium of the renal tubule, which is associated with AKI [45]. Increased urinary carnosine in AKI may possibly representing a protective metabolic role from the free radicals and help to maintain acid base balance. Also the preventing effect of carnosine on ischemic AKI is probably through the suppression of enhanced renal sympathetic nerve activity induced by ischemia/reperfusion [46]. Previous studies in adults with AKI have also confirmed our results as, they documented altered plasma amino acids concentrations in these patients and that the levels of urinary polyamines and some single amino acids were increased before alterations in kidney histology were observed, implying a disturbed protein metabolism in these patients [47].With understanding of the process where different metabolites work within the regions of the kidney, the location of damage can be determined based on the altered levels of metabolites [48]. Altered glucose metabolism is a major metabolic disturbance in AKI [49]. In this study, the urinary glucose concentration was higher in AKI neonates as compared with no-AKI ones. Glucosuria was found to be a marker of proximal tubular dysfunction, which resulted in defective reabsorbition [43].Betaine is a small N-trimethylated amino acid, that is synthesized from choline by choline dehydrogenase and betaine- aldehyde dehydrogenase in gut mucosa, liver and kidney [50]. It is involved in cell volume homeostasis as well as in cell protection against oxidative stress, as it has an inhibitory effect on nitric oxide release. It plays a role in the synthesis of carnitine and helps to protect the kidney from damage [51]. Betaine as one of the renal osmolytes is essential for renal medullary health, in protecting cells and proteins from the harsh osmotic gradients needed to produce concentrated urine, so it is regulated by osmolar stress [52], [51]. Our data showed that several metabolic pathways are influenced in neonatal AKI. In particular, we noted significant elevations in the amounts of urinary Betaine, the presence of which had a significant statistical impact on the differentiation of AKI neonates from those without the disease. As Betaine has anti-osmotic and anti-oxidant properties [53], increased urinary concentrations of this compound may have a cytoprotective effect.Allantoin, as a product of the purine degradation pathway, which result from degradation of purine nucleobases, it also results from oxidation of uric acid [54]. It was shown that after ischemia / reperfusion in kidney tissue in AKI, allantion a well known marker of oxidative stress was increased, so it can be used as a marker of oxidative stress in this condition [16]. In our study, we found allantoin to be present in increased amounts in samples from AKI neonates as compared with no-AKI ones, it may be increased to counteract oxidative stress. The nucleotide metabolism was found to be disturbed in ischemic injury and purine metabolism also seems to play a crucial role during AKI [55]. Accumulation of purines may result from the cellular destruction following AKI and the degradation of high-energy phosphate compounds [56]. Acetic acid is a simple monocarboxlic acid, when bound to coenzyme A, it is central to the metabolism of carbohydrates and fats [54]. Our results showed higher levels of urinary acetic acid in AKI neonates in comparison with no- AKI ones. As, AKI is an inflammatory condition, this was matched with Nevedomskaya et al. [57],who found that, higher acetate levels were associated with the presence of urinary tract infection.In addition we found that, the urine PH of the AKI neonates was significantly different from no- AKI ones, this was in agreement with Mercier et al. [37], who showed similar results. Some studies demonstrated that Netrin-1 could be secreted into urine within 1 h of renal injury, and increased to 30–40 times 3 h later, which was earlier than the elevated sCr and urea. Its level drops to near normal once renal function recovers while sCr remain elevated until 24 h after reperfusion, suggesting that NTn-1 may serve as both an early injury marker and a prognostic marker of renal recovery [58]. Our results confirmed this findings as, we found highly significant increase in serum level of NTn-1 in AKI as compared to no-AKI neonates, and its level was positively correlated with sCr. This result was consistent with Cao et al. [59], who showed similar result and thus, urinary NTn-1 can be used as indicators for the early diagnosis of AKI. Moreover, Ramesh et al. [60] found higher levels of NTn-1 in urine samples from patients with drug-induced AKI, ischemic AKI, sepsis-induced AKI and radiocontrast-induced AKI in comparison with healthy controls. They concluded that NTn-1 can serve as universal biomarker for AKI by demonstrating increased levels in patients with various forms of AKI. Our results were also in consistent with previous study done by Tu et al. [61], who found that urinary NTn-1 levels were significantly higher in AKI patients as early as 1 hr, peaked at 3–6 h and remained elevated up to 48 hrs, while sCr levels elevated 24 h later, which indicated that urinary netrin-1 is a biomarker for the early diagnosis of AKI.By contrast, Oncel et al. [62] reported that the level of NTn-1 was similar in both AKI and no-AKI neonates, this could be explained by variation between the two studies as regard gestational age, disease duration and may be the sample size.
5. Conclusions
- Our data suggested that using metabolic profile measurement with other markers for AKI may lead to a more sensitive, specific, and accurate predictive capability of AKI in preterm infants. Metabolomics hold promise for early diagnosis, increased choice of therapy and the identification of new metabolic pathways that could potentially be targeted in kidney disease. Urinary Netrin-1 is a promising early upregulated biomarker for AKI, and its utility may be of particular importance due to early detection of AKI and allow for appropriate and timely interventions which may significantly decrease morbidity and mortality related to AKI in the future.
Study Limitation
- Small sample size, measurements of metabolic profile and NTn-1 were done once, while measurement of more than one sample will provide better results. What is already known on this topic: In the previous studies, the researchers have identified novel biomarkers that allow for the earlier identification of neonates with AKI. What this paper adds. Using metabolic profile measurement with other markers for AKI may lead to a more sensitive, specific, and accurate predictive capability of AKI in preterm infants.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML