-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2018; 8(4): 71-78
doi:10.5923/j.ajmms.20180804.04

Effects of Gestational Age on Serum Levels of Some Antioxidant Micronutrients in Rural Areas of South-Eastern Nigeria
Sylvester O. Ogbodo1, Antoinette NC Okaka2, Uchenna I. Nwagha3, Emmanuel I. Nwobodo4
1Dept. of Medical Biochemistry, Faculty of Basic Medical Sciences, College of Medicine, Enugu State University of Science and Technology, Enugu, Nigeria
2Dept. of Applied Biochemistry, Faculty of Biosciences, Nnamdi Azikiwe University, Awka, Nigeria
3Dept. of Physiology, Obstetrics and Gynecology, College of Medicine, University of Nigeria, Enugu Campus, Nigeria
4Dept. of Biochemistry, Faculty of Natural Sciences, Chukwuemeka Odumegwu Ojukwu University Uli, Anambra State, Nigeria
Correspondence to: Sylvester O. Ogbodo, Dept. of Medical Biochemistry, Faculty of Basic Medical Sciences, College of Medicine, Enugu State University of Science and Technology, Enugu, Nigeria.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Oxidative stress in pregnancy reduces immunity and predisposes pregnant women to many diseases, prompting the need for antioxidants’ supplementations. This cross-sectional study was undertaken to make more comprehensive evaluation of levels of antioxidant micronutrients in different trimesters in uncomplicated pregnancy in rural setting and establish correlation between gestational age and antioxidant micronutrients.Subjects were 195 pregnant women in different trimesters, attending antenatal clinics for the first time in their current pregnancies while controls were 50 age-matched, non-pregnant women from the same environment. Vitamins (A, C and E) were estimated spectrophotometrically while trace elements (Cu, Mn, Se and Zn) were determined using atomic absorption spectroscopy.Our results showed that there were statistically significant changes in the values of most of the antioxidant micronutrients from the patients when compared with those from the controls. Only copper did not show significant change (p=0.141), manganese increased significantly (p=0.001) while others decreased significantly (p<0.001 each) in pregnancy. There was no correlation between these parameters and gestational age except vitamin C that showed negative correlation (r2=0.968; p=0.016). The results showed that antioxidant micronutrients decrease significantly during pregnancy in rural areas. We advocate for governments’ intermittent intervention programmes for antenatal care in these rural areas to augment their antioxidants’ supplementations.
Keywords: Antioxidant micronutrients, Gestational age, Rural areas
Cite this paper: Sylvester O. Ogbodo, Antoinette NC Okaka, Uchenna I. Nwagha, Emmanuel I. Nwobodo, Effects of Gestational Age on Serum Levels of Some Antioxidant Micronutrients in Rural Areas of South-Eastern Nigeria, American Journal of Medicine and Medical Sciences, Vol. 8 No. 4, 2018, pp. 71-78. doi: 10.5923/j.ajmms.20180804.04.
Article Outline
1. Introduction
- Various biochemical activities within the body generate a lot of reactive species (free radicals) including reactive oxygen species (ROS), reactive nitrogen species (RNS) and reactive chloride species (RCS). Exogenous sources of these free radicals include unhealthy food habits, infections and stress conditions like pregnancy, among others. Under physiological conditions, these free radicals are used by the body in fighting infections, induction of apoptosis, regulation of vascular tone, regulation of gene expression and cell differentiation [1-3]. Some deleterious effects caused while performing these biological functions are ameliorated by regenerating or repair system. However, when the rate of generation overwhelms the regenerating and repair systems, oxidative stress ensues, breaking down cellular molecules, which require the antioxidants’ interventions. This oxidative stress weakens immune functions and enhances aging process, as well as causing many diseases such as cataracts, some cancers, heart disease, arthritis and many other chronic conditions [4]. In addition, it causes development of preeclamptic toxemia in pregnancy and other uncountable perinatal and maternal illnesses that lead to many untoward pregnancy outcomes [5, 6].Pregnancy is a physiological stress condition that reduces immunity [7] and greatly predisposes pregnant women to infections and other diseases. Under this condition, generated free radicals cause oxidative stress and result in absolute need for antioxidants to avoid adverse effects on pregnancy. Low blood levels of some of these antioxidants have been implicated in preeclampsia and abnormal fetal and childhood development [3, 6]. Particularly, zinc and selenium deficiencies were found to cause frequent abortion, prolonged gestation, teratogenic effects, stillbirth, difficult parturition, toxemia and low birth weight [8]. Other researchers [9-11] have emphasized the involvement of micronutrients deficiencies in many complications of pregnancy, and antioxidants roles in good pregnancy outcomes and excellent lactation. Adequate intake of antioxidants during pregnancy was found to reduce cell death and other malformations, save brain cells of off-springs of women exposed to ethanol, and reduce death due to cerebral malaria in mice [12]. Unfortunately, exact macro- and micronutrients needs of pregnant women in the rural areas have not been adequately evaluated [13], and doing so will help to assess and identify areas of improvement. The assessment is by establishing the basic serum levels of these antioxidants in apparently normal pregnancy and at different trimesters so that deficient ones can be targeted for supplementation during pregnancy. From the foregoing, and considering the fact that the micronutrient status of a pregnant woman is an important determinant of fetal growth and survival, as well as life immediately after birth [14], information on the relative concentrations and therefore requirements of these micronutrients during pregnancy is very vital and inevitable. The aim of this present study was therefore to establish the basic levels of some of these antioxidant micronutrients (vitamins and trace elements) in uncomplicated pregnancy and their correlations with gestational age in our rural areas to help our obstetricians and other pregnancy-care givers in the rural areas to make lasting decisions for our pregnant women. The knowledge will also be useful for pediatricians for early care of the resultant infants, especially low birth weight infants, since the principal cause of micronutrients deficiencies in these infants is their preceding deficiencies during gestation [15].
2. Materials and Methods
2.1. Study Areas
- The study areas included two rural communities each from Ebonyi and Enugu States of Nigeria. These areas have similar environmental, religious and socio economic settings. For instance, inhabitants are mainly artisans and low class civil servants; their markets are controlled by the villagers, except few itinerant traders; farmers are generally subsistent farmers; their staple foods are cassava, yam, rice and corn, which are consumed daily; malaria endemicity in these areas are similar – 59.9% in Ebonyi State [16] and 58.4% in Enugu State [17]; public water supply is mainly from commercial water vendors – tanker drivers; inhabitants are mainly Christians and African traditional religionists, and their Health Centers are manned by few Nurse/Midwives and Community Health Extension Workers with occasional visits by qualified medical doctors – mainly on antenatal days.
2.2. Subjects
- Our subjects were 195 pregnant women (out of 315 initially enlisted) who were attending the antenatal clinics of the Health Centers in the study areas for the first time during their current respective pregnancies. They were aged between 18 and 40 years. This number was made up of 51 in first trimester (1 – 13 weeks), 66 in second trimester (14 – 28 weeks) and 78 in third trimester (29 weeks to term). Subjects in their first trimesters were newly married primigravidae and pregnant spinsters who presented in the health centers with amenorrhea, thus confirming earlier reports that women in developing countries hardly go for antenatal care within their first trimester [16, 18]. From ultrasound reports, all the subjects were singleton. Our control subjects (50) were age-matched, non-pregnant, unmarried and apparently healthy women, who were not menstruating at the time of sample collection. All the subjects were primary and secondary school teachers, local government workers and health workers from the study areas, with similar dietary habits and educational levels. Ethical clearance for this study was obtained from Health Research Ethics Committee of University of Nigeria Teaching Hospital, Enugu. Additional informed consents were sought and obtained from the subjects. After counseling, they willingly consented to be enrolled for the study and later willingly filled the questionnaires. The subjects were divided into 4 groups – controls, first trimester, second trimester and third trimester.
2.3. Exclusion Criteria
- Some of the multigravidae in their second and third trimesters in our study areas were already taking routine antenatal drugs – vitamin B complex, Folic acid, Fesolate (iron tablets) and vitamin C or multivitamin capsules, before coming to register for antenatal care. This group was therefore excluded from the study. Those in their first trimesters were either newly married women or spinsters who presented with amenorrhea and/or undefined fever and missed menstrual period. They were counseled and tentatively enlisted for the study, investigated for pregnancy and other fever-causing conditions and those found to be pregnant only were recruited while others were delisted. Other exclusion criteria were history of repeated caesarian sections and habitual alcohol consumption, including the locally tapped and constituted palm wine. All subjects were screened for malaria infections and chronic diseases like diabetes, hypertension and HIV infections, and those found positive were delisted.
2.4. Dietary Index
- The dietary indices of the study areas were calculated from the preliminary investigations using both 24-hour dietary recall and estimated food records as earlier reported [19]. Before the study, randomly selected and counseled twenty (20) women from each of the study areas were interviewed on their food intake. Each person was asked to recall the food consumed within the previous 24 hours – including raw, locally prepared and industrially processed foods. From this, the amount of nutrients intake within the 24 hours were calculated based on the known compositions of commonly eaten foods in Nigeria [19]. This was used to estimate the food records/nutritional indices of these areas before the commencement of the study. This preliminary work showed that the study areas have similar dietary indices. Later, further calculations were made from the 7-day retrospective dietary records given by our subjects in answer to a section of the questionnaires. This also gave similar dietary indices, thus confirming the close relationship among the study areas in terms of nutrient intake.
2.5. Sampling
- A total of 8.0ml of venous blood was collected from each subject into a chemically clean and dry glass test tube and transported in a sample pack wrapped with black polythene to protect the analytes from direct sunlight. The sample was allowed to stand for 30 minutes at room temperature to clot and retract, and then centrifuged for 10 minutes at 3000 rpm. The serum obtained was aliquotized into two and stored frozen at -20°C until needed for analysis. Vitamins were usually estimated within 48 hours of collection while the trace elements were estimated within two weeks of collection.
2.6. Laboratory Analysis
- Vitamin A was estimated by method recommended by International Vitamin A Consultative Group (IVACG) in 1982 as modified by Ene-Obong et al [20], vitamin C was estimated by a modified 2,4-dinitrophenylhydrazine method [20] while vitamin E was estimated using method of Pearson [21]. Trace elements – Copper (Cu), Manganese (Mn), Selenium (Se) and Zinc (Zn), were estimated using atomic absorption spectroscopy (Buck Scientific Spectrophotometer Model 205, East Norwalk, Connecticut, USA).
2.7. Statistical Analysis
- Graph pad prism version 5.03 was used for the statistical analyses. t-test was used to compare the mean values obtained from both patients and controls while 2-tailed Pearson correlation was used for the correlation studies.
3. Results
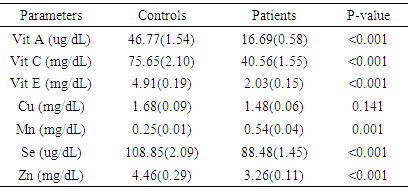
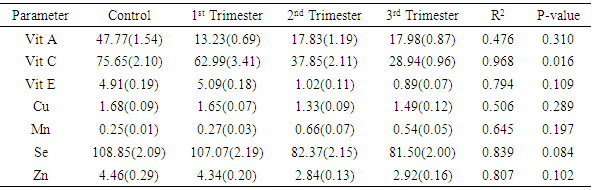
- The average age of the subjects was 24.9years while that of the controls was 24.1years. Results for controls, first, second and third trimesters respectively were 46.77 ± 1.54µg/dL, 13.23 ± 0.69µg/dL, 17.83 ± 1.19µg/dL, and 17.98 ± 0.87µg/dL for vitamin A; 75.65 ± 2.10mg/dL, 62.99 ± 3.41mg/dL, 37.85 ± 2.11mg/dL, 28.94 ± 0.96mg/dL for vitamin C; 4.91 ± 0.19mg/dL, 5.09 ± 0.18mg/dL, 1.02 ± 0.11mg/dL, and 0.89 ± 0.07mg/dL for vitamin E; 1.68 ± 0.09mg/dL, 1.65 ± 0.07 mg/dL, 1.33 ± 0.09 mg/dL and 1.49 ± 0.12 mg/dL for copper; 0.25 ±0.01mg/dL, 0.27 ±0.03mg/dL, 0.66 ±0.07mg/dL and 0.54 ±0.05mg/dL for manganese; 108.85 ± 2.09µg/dL, 107.07 ±2.19µg/dL, 82.37 ± 2.15µg/dL and 81.50 ± 2.0µg/dL for selenium, and 4.46 ± 0.29mg/dL, 4.34 ± 0.20mg/dL, 2.84 ± 0.13mg/dL and 2.92 ± 0.16mg/dL for zinc. Table 1 shows the mean concentrations and standard deviations (in parenthesis) of all the antioxidant micronutrients in patients and control, showing statistical changes in pregnancy. Only copper did not show significant change (p=0.141), manganese showed significant increase (p=0.001) while others showed significant decrease (p<0.001 each) in pregnancy.
|
|
|
|
4. Discussion
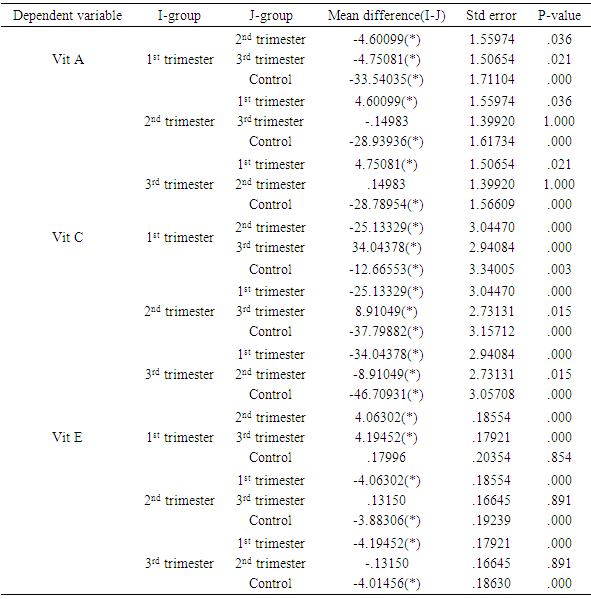
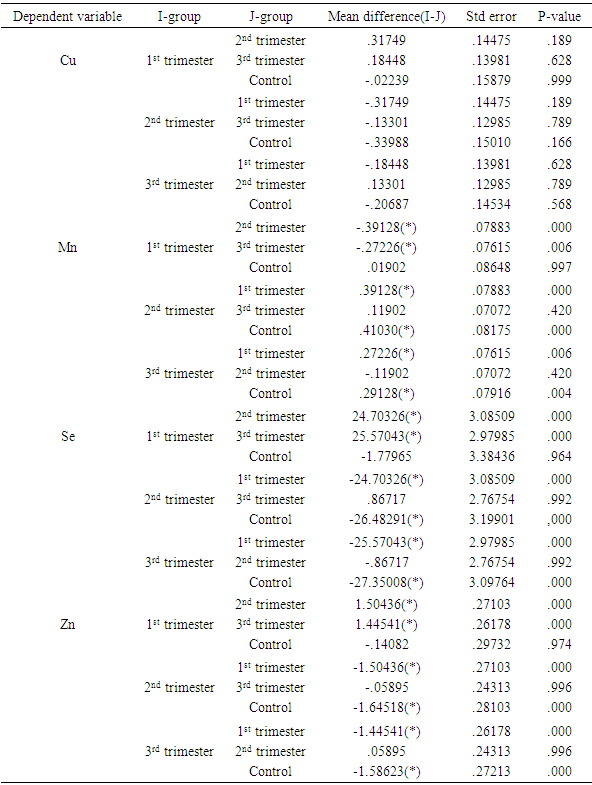
- Poor nutrition and inevitable macro- and micronutrients deficiencies are known to play important roles in the pathogenesis of malaria and malarial anemia, especially in immuno-compromised individuals like pregnant women and under-aged children [22, 23]. This ensures that people from rural areas with high socio-economic burden and attendant under-nutrition, are constantly bombarded with persistent infections and infestations as well as untoward pregnancy outcomes.The mean serum concentrations of vitamins A and C obtained from the controls of this study are consistent with earlier reported reference values for these vitamins [24]. However, the vitamins decreased significantly during pregnancy and in all the trimesters, with vitamin C showing significant negative correlation with gestational age (r2=0.968; p=0.016). The significant decrease during pregnancy may be related to the fact that the natural sources of these vitamins (fruits and vegetables) are rarely consumed in these areas, in addition to increased demand from the fetus. For instance, it was noticed that green vegetables that are normally produced in large quantities in these areas are rarely consumed; instead they are transported to urban areas for paltry financial returns. Vitamin A is essential for vision, immune development and some other metabolic processes [25], especially in early life. Though a large cluster randomized trial of vitamin A supplementation during pregnancy did not show much effects on neonatal mortality or mortality in the first six months of life [26], the present study supports a sub-analysis [27] suggesting a trend towards an effect. Therefore, this sharp drop in value, which was maintained throughout gestation, portends danger to the fetuses and expectant mothers alike, especially in terms of susceptibility to infection and retardation of growth and cell differentiation. From the results of this study, vitamin A supplementation in the study areas is very necessary and inevitable. However, supplementation must follow earlier advice that the initiation of vitamin A supplementation should be carefully examined in each case according to the risk-to-benefit ratio, resting final decision on the account of estimated vitamin A status of the woman, the availability of vitamin A-rich foods in her diet, and whether supplementation can be supervised [28]. The negative correlation of vitamin C with gestational age is consistent with earlier studies [18, 29, 30]. Vitamin C plays crucial roles in metabolism within the body including prevention of iron-deficiency anemia [18, 31], and modulation of several components of the human immune system, helping the mother and the baby to fight infections. Adequate intake of vitamin C reduces the risk of rupturing chorio-amniotic membrane, thereby reducing infection and premature delivery; and also regenerates other antioxidants especially vitamin E [32], making its adequate concentration throughout gestation a high necessity. This result shows that the benefits of adequate vitamin C concentrations during pregnancy is seriously lacking in the study areas and need to be addressed in terms of supplementation and nutrition education during antenatal visits. Unlike vitamins A and C, the mean serum concentration of vitamin E from the controls of this study was higher than reference value earlier reported in humans [24]. However, like vitamin A, there was significant lower level in pregnancy and non-significant correlation with gestational age. This result disagrees with earlier report of 12.36% increase in vitamin E concentration during pregnancy [29]. Though there is limited information about its function in human reproduction, vitamin E is involved in several biological processes including immune functions, growth and cell differentiation, and therefore very important in early embryogenesis [33]. Moreover, its deficiency in pregnant rats was found to have terminated pregnancies within 10 days due to fetal death and resorption of uterine contents [34], making its deficiency during pregnancy a great risk and supplementation a necessity.For the trace elements, the serum concentrations from the controls of this study showed varying deviations from earlier reported reference values [35]. While the concentrations of copper and selenium in the controls were consistent with those earlier values, manganese was higher while zinc was lower. On the other hand, copper decreased non-significantly (p=0.141) in pregnancy, with non-significant correlation with gestational age. This is consistent with a recent study within the same environment [36] but at variance with earlier studies in other environments [37-39]. These variations may be related to the differences in both the copper content of the soil and the social status of the subjects from the different areas or possible initial intake of antenatal routine drugs before collection of samples in the previous studies. Manganese increased significantly during pregnancy though without significant correlation with gestational age. This agrees with earlier studies on the same class of patients [40-42]. Takser et al [42] were of the opinion that lifestyle and environmental factors may interfere with the delicate balance and homeostatic mechanisms required to maintain manganese at optimal levels for physiological changes during pregnancy. However, since inadequacy is not the problem here but increase, pregnant women need to be monitored for liver impairment and iron or calcium deficiency known to be involved in manganese toxicity [43]. Both selenium and zinc decreased significantly in pregnancy though without significant negative correlation with gestational age. The selenium concentrations from this study are consistent with earlier studies on serum and red cell selenium concentrations in pregnancy [44, 45], which also demonstrated negative correlation between red cell selenium and recurrent pregnancy loss/habitual miscarriage. Selenium, among other things, decreases oxidative stress and inflammation and enhances hormone production and immune functions [15, 46]. Its deficiency has been reported in miscarriages and pre-eclampsia [47, 48], hence, maintenance of adequate serum concentration of this trace element throughout gestation, especially in the rural areas, should be a priority in antenatal care. Low serum zinc in our control is consistent with earlier report that zinc deficiency is prevalent in the developing world where only cereals are consumed by the populace as staple food [49]. Its decrease in pregnancy is also consistent with those earlier reported from different parts of the world including Nigeria, Zaire, Spain, Kuwait and India [36, 37, 39, 50, 51]. Its progressive decrease over gestation, though not statistically correlated, also agrees with earlier report that zinc requirement in the third trimester of pregnancy is approximately twice that of non-pregnant women [52]. The deficiency at term can cause many untoward pregnancy outcomes [51, 53], and also lead to zinc deficiency in infants, especially premature infants who were deprived of incorporated fetal zinc, impairing innate and acquired immunity development as well as causing growth retardation for these infants.The deficiencies of these antioxidant micronutrients in this study supports earlier call for the establishment of reference values of these parameters in normal pregnancy in each locality [54] to help obstetricians, pediatricians and other healthcare-givers deliver optimum services to their patients. Though such results are not entirely unexpected in rural areas of a developing country like ours where under-nutrition is the other of the day, the situation is however not comfortable for such areas with unprecedented malaria endemicity [16, 17], considering that these antioxidants should be the first line of defense to these infections before the institution of chemotherapy, where they are available and/or affordable.
5. Conclusions
- Our results showed wide range deficiencies of studied antioxidant micronutrients in pregnancy, except manganese. There was also continued decrease of the antioxidants as pregnancy progressed, though without significant correlations between these antioxidants and gestational age except vitamin C. We hereby re-emphasize earlier call for intermittent antenatal drugs interventions by governments, government agencies and non-governmental organizations to help rural duelers meet drug supplementations during pregnancy [55]. We are also of the opinion that governments of these developing countries should institute proper healthcare policies that will take adequate care of the most vulnerable set of people, particularly the pregnant women and infants. This will replace the occasional free treatment programmess that will only work on papers but cannot be implemented for the benefit of those they were meant for.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML