-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2018; 8(3): 43-48
doi:10.5923/j.ajmms.20180803.02

Fetomaternal Immunoglobulin among Mothers in University of Port Harcourt Teaching Hospital
Kenneth S. Ordu, Clinton D. Orupabo, Progress D. Victor, Rachael S. Jafaru
Department of Human Anatomy, College of Medical Sciences, Rivers State University, Rivers State, Nigeria
Correspondence to: Clinton D. Orupabo, Department of Human Anatomy, College of Medical Sciences, Rivers State University, Rivers State, Nigeria.
| Email: |  |
Copyright © 2018 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

During pregnancy, there is decrease in maternal immunoglobulin levels due to suppression of maternal immunity in order to prevent rejection of the foetal allograft.Hypertension influences the values of maternal immunoglobulins thereby affecting pregnancy outcome. This study was therefore conducted to determine the feto – maternal serum immunoglobulin levels in hypertensive and healthy mothers in University of Port Harcourt Teaching Hospital. Sixteen (16) pregnant women were randomly recruited for this study; 6 hypertensive and 10 healthy mothers. Serum samples of both mother and foetal umbilical cord were collected and analyzed for three immunoglobulins: IgG, IgM and IgA. The mean maternal IgG levels of mothers with hypertension and those in a healthy state were 968.33±163.27 mg/dl and 942.20±103.06 mg/dl respectively while their corresponding mean foetal IgG values were 1121.67±171.16 mg/dl and 987.00±120.74 mg/dl. Also, the mean maternal IgM levels of mothers with hypertension and those in a healthy state were 94.00±44.38 mg/dl and 61.90±17.95 mg/dl respectively while the mean maternal IgA levels of mothers with hypertension and those in a healthy state were 134.67±27.00 mg/dl and 92.60±34.28 mg/dl respectively. The result showed higher IgG, IgM and IgA immunoglobulins levels in hypertensive than non-hypertensive mothers. The levels of the mean foetal IgA and IgM were significantly low compared to foetal IgG, though higher in healthy mothers. This study had shown that hypertension increases the serum levels of maternal immunoglobulin thereby causing deleterious effects on the growing foetus.
Keywords: Immunoglobulins, Hypertension, Pregnancy, Foetus
Cite this paper: Kenneth S. Ordu, Clinton D. Orupabo, Progress D. Victor, Rachael S. Jafaru, Fetomaternal Immunoglobulin among Mothers in University of Port Harcourt Teaching Hospital, American Journal of Medicine and Medical Sciences, Vol. 8 No. 3, 2018, pp. 43-48. doi: 10.5923/j.ajmms.20180803.02.
1. Introduction
- Immune responses play an important role in various reproductive processes including ovulation, menstruation, pregnancy and parturition. Pregnancy may be considered as a state where the foetus exists as a well-tolerated semi-allograft since they generally differ from the mother by having a major inherited part of their genetic contribution from their paternal end. Hence suppression of maternal immune responses may be one of the factors contributing to continuation of pregnancy [1]. It is possible that the foetus escapes the process of rejection due to depression of maternal immune responses. Conversely, a disturbance in immunologic tolerance may lead to complications during pregnancy [2]. This was first recognized by Medawar in 1953 [3] when the concept of the foetal allograft was presented in order to explain the immunological relationship between mother and foetus. Since then, the immunology of pregnancy has been the leading subject within reproductive immunology research. It has also become a relevant aspect in the practice of clinical anatomy and aims to curb the prevalence of perinatal and maternal complications during and after pregnancy. Yet, the question of why the semi-allogeneic fetus is not rejected by the mother remains unresolved. This particular study provides an update on current knowledge on the subject of the so-called immunological paradox of pregnancy. Immunoglobulins (Igs) can be defined as glycoprotein molecules also called antibodies (Abs) that are produced in response to foreign substances entering the living body. These substances are either antigens (Ag) or immunogens (viruses, bacteria, or toxins etc). They produce this response by binding to these recognized antigens and forming antigen-antibody complexes resulting in Ag elimination and protection of the body of the host. Igs are produced by the lymphocytes and are found in fraction of blood called gamma globulin [4]. The production of these antibodies (Igs) is the main function of the humoral immune system [5]. Antibodies are usually secreted by B cells of the adaptive immune system, mostly by differentiated B cells called plasma cells. Antibodies can occur in two physical forms; a soluble form that is secreted from the cell to be free in the blood plasma, and a membrane-bound form that is attached to the surface of a B cell and is referred to as the B-cell receptor (BCR). The BCR is found only on the surface of B cells and facilitates the activation of these cells and their subsequent differentiation into either antibody factories called plasma cells or memory B cells. These would survive in the body and remember that same antigen to aid a faster and more effective response by B cells in the future, if there is same exposure to the same antigen [6]. There are five main classes of heavy chain constant domains (CH). Each class defines the IgM, IgG, IgA, IgD, and IgE isotypes. The CH genes for each isotype are aligned on the same transcriptional orientation on the human chromosome 14. Isotypes differ in a number of properties; including size, complement fixation, FcR binding, and isotype response to antigen. The choice of isotype is dependent on the antigen itself and the signaling pathways that are activated, as well as the local microenvironment [7]. For this research purpose, only IgA, IgG, IgM will be discussed.The goal of every pregnancy is a positive pregnancy outcome and the maternal immune system contributes immensely to a successful foetal development after conception. This occurs by the production of Immune cells which accumulates in the human endometrium at the time of decasualization. They play critical and diverse roles at the maternal–foetal interface, which includes implantation, placental development, and immunity against infectious diseases [8]. Immunology in pregnancy is not only for foetal development, it also contributes to maternal sustenance to continue by providing host defense against infection. Furthermore, other clinical immunological studies have demonstrated that children of mothers exposed to certain infectious organisms during pregnancy have significantly higher frequencies of neurological disorders [9-16] including schizophrenia and autism spectrum disorders. The placenta is the highly specialized organ of pregnancy that supports the normal growth and development of the fetus. It could also be described as a flattened circular organ in the uterus of pregnant mammals that nourishes and maintains the foetus through the umbilical cord [17]. More so, after the first week of implantation the placenta gradually begins to develop from the development of the extra-embryonic membranes which begins at the moment of the differentiation of the blastocyst cells into an embryoblast and a trophoblast.The embryoblast forms the later embryo and the trophoblast, the components of the embryonic appendage organs. The formation of the placenta is induced by the syncytiotrophoblast of the blastocyst, which triggers the decidual reaction of the uterine wall. This change in the endometrium also depends on the stimulation by the hormones released by the ovary and placenta. The differentiation of the placenta begins with the formation of lacunae in the syncytiotrophoblast that are filled with maternal blood, which stems from the spiral arteries. The placenta connects the developing foetus to the uterine wall through the umbilical cord. It allows nutrient uptake, provide thermo-regulation to the fetus, waste elimination, and gas exchange via the mother’s blood supply. It also helps to fight against internal infection and produce hormones to support pregnancy [17]. IgG transfer from mother to fetus also depends amongst other factors on the gestational age of the mother. This transfer begins as early as the 13th week of gestation, and transport happens in a linear fashion as the pregnancy progresses, with the largest amount transferred in the third trimester [18]. Hypertension is the most common medical problem encountered during pregnancy, complicating 2-3% of pregnancies. Hypertension is defined as either a systolic blood pressure of 140 mm Hg or greater, a diastolic blood pressure of 90 mm Hg or greater, or both. Hypertension is considered mild until diastolic or systolic levels reach or exceed 110mmHg and 160mmHg respectively [19]. The occurrence of hypertension increases risks for preterm birth, low birth weight and small for gestational age (SGA) birth as well as for low APGAR scores, intrauterine fetal death(IUFD), intrauterine growth restriction (IUGR). This current research is to evaluate the effect of hypertension in pregnancy on the various transplacental immunoglobulin (IgG, IgM, and IgA) levels in the hypertensive mothers and their foetus (es) in contrast with healthy pregnant mothers and their foetus (es). It is also to determine if there is correlation or variation between these immunoglobulin levels of the mother and the foetus (es) of each group. The data obtained could contribute to being a template for further researches of fetomaternal tolerance and even give a predicting marker of the risk of foetal development.Rote et al, [20] identified IgG and IgM on the surface of maternal platelets in both autoimmune thrombocytopenia (ATP) and pregnancy-induced hypertension (PIH). IgG was also found on the umbilical cord platelets of patients with ATP and PIH, whereas IgM was only found on the umbilical cord platelets of patients with PIH. The possible maternal or foetal origins of these umbilical cord blood immunoglobulins were investigated by immunoblot analysis of antibodies in paired maternal and umbilical cord blood sera of ATP and PIH patients. Maternal sera contained IgG and IgM antibodies which reacted with several platelet proteins. However, a large amount of patient-to-patient variation was observed in the specific antigens that were identified. Analysis of paired maternal and umbilical cord sera from patients with ATP or PIH showed identical patterns of antigen specificity, which suggested that the IgG antibodies in the foetal circulation were of maternal origin. Circulating IgM antibodies were not observed in the umbilical cord sera of ATP patients. The umbilical cord sera of PIH patients, however, contained IgM antibodies that reacted against a variety of platelet antigens. In addition, most umbilical cord sera from PIH patients had identical patterns and relative intensities of reactivity, which differed from the patterns observed in the paired maternal sera. Antiplatelet IgM in the umbilical cord blood of PIH patients, therefore, appears to be a product of the foetal immune system. Amah-Tariah et al, [21] carried out a study on serum immunoglobulin changes in pregnancy complicated with pre eclampsia and diabetes in Nigerian women and compared it to both healthy pregnant and non-pregnant subjects resident in Port Harcourt. Venous blood samples obtained were analyzed for IgG, IgM, and IgA with the turbidimetric immunoassay method using an automated chemical analyzer. They observed that the values of both IgG and IgM were significantly higher while the values of IgA were significantly lower amongst the non-pregnant subjects. Furthermore, the healthy pregnant subjects were found to have significantly higher values of IgG and lower levels of IgM compared to subjects with pre eclampsia and gestational diabetes (GDM). Values of IgA were significantly higher in all pregnant groups compared to the non-pregnant subjects but highest in the GDM. Also during the course of gestation, a graded decrease in the mean values of all three immunoglobulin classes was observed in the healthy pregnant group from the 1st to 3rd trimester. However, whereas a graded increase in mean IgA and IgM were observed in subjects with pre eclampsia across trimesters, mean IgG showed a similar graded but significant decrease. Also, the mean IgG decreased significantly in the pregnant diabetic in the 2nd trimester, but that of IgM significantly increased in the 2nd trimester. Another study by Ahsan et al, [22] documented the following results with mean serum IgG concentrations as 7.95±0.69g/L, 7.66±0.46g/L and 7.10±0.61g/L; IgA concentrations were 4.07±0.32g/L, 4.10±0.32g/L and 3.65±0.32g/L; and IgM concentrations were 2.01±0.21g/L, 2.92±0.29g/L and 2.16±0.16g/L in pre-eclampsia, eclampsia and normotensive pregnant subjects respectively. Amah-Tariah et al, [23] in another similar study reported that IgG and IgM levels were significantly lower and IgA values significantly increased in the pregnant subjects compared to the non-pregnant subjects. Also in subjects with malaria in pregnancy, IgG and IgM levels were consistently and significantly low. Mean IgA values were found to be consistently higher in both pregnant malaria and HIV subjects and being the lowest amongst the healthy pregnant subjects. Obiandu et al, [24] worked on the levels of serum immunoglobulins in apparently healthy children and adults in Port Harcourt, Nigeria and observed that female children were found to have significantly lower mean values of IgA compared to male children. Female adults had significantly higher mean values of IgG and significantly lower mean values of IgA compared to male adults. No significant differences were observed in the mean values of any of the various types of immunoglobulin between adults and children. Salimonu et al, [25] documented on Serum immunoglobulin levels in normal, premature and postmature newborns and their mothers. They noticed a progressive increase in mean IgG levels as the gestational age increases. The mean IgG level of infants of 32--36 weeks' gestation was lowest while the maternal IgG levels were generally higher than those of the cord sera. The mean IgA and IgM levels of the cord sera were low. There were no significant differences between the mean IgA and IgM levels of newborns in different gestational age groups, even when their mothers showed variations in mean IgA and IgM levels. There was no detectable level of IgD in the cord sera. Malek et al, [26] reported on the concentration of IgG and its four subclasses and of IgA in the sera using ELISA method. Both IgG and IgA decreased throughout pregnancy to a level of 60–70% of the initial concentration in early pregnancy. They observed that during pregnancy the human placenta developed a specific transport mechanism for IgG and that there are differences for the four subclasses with preferential transfer of IgG1 while the slowest transfer is seen for IgG2. This present study is aimed at comparing the levels of serum immunoglobulin between hypertensive and healthy pregnant mothers in University of Port Harcourt Teaching Hospital.
2. Materials and Method
- This study is a prospective cross-sectional study that is hospital based and carried out on hypertensive and healthy pregnant women in the University of Port Harcourt Teaching Hospital, Rivers State for the period of 1 month. The subjects were selected through convenient sampling method. The research investigated the serum Immunoglobulin levels in hypertensive and non-hypertensive pregnant women and their foetus (es) at term. The antenatal records of the pregnant subjects were used as well as blood samples from hypertensive and healthy pregnant mothers and the corresponding umbilical cord of their foetus. A total number of 16 paired blood samples (maternal and foetal sera) of the pregnant women (6 hypertensive pregnant women and 10 healthy women as control) was obtained through convenient sampling method. All pregnant patients with elevated blood pressure readings above 140/90 (mm/hg) and pregnant patients with non-elevated blood pressure above 140/90 (mm/hg) were selected. Pregnant patients with autoimmune diseases, HIV, and other conditions like diabetes were not selected. 5ml of venous blood was carefully collected from an ante-cubital vein with the maternal subject comfortably seated. Also same volume of blood was withdrawn from the corresponding umbilical cord of the foetus of the maternal subjects with a 5ml syringe and needle. The blood collected was transferred into anticoagulant sample bottles and appropriately labeled. The samples were centrifuged, serum obtained and stored at -20°C until ready for immunoglobulin assay. All assays were done within two weeks of blood collection. Serum levels of immunoglobulins A, G and M was determined on the withdrawn sample using the immune turbidimetric assay method with a fully smart automated clinical chemistry analyzer (Biochemical Systems International Srl, Italy), as well as Cromatest immunoglobulins A, G and M kits (Barcelona Spain). Preparation of reagent was done using the standard start method. This involved dilution of a standard powder of the reagent (SP-NORMOTROL-a lyophilized human-based control serum) with 5ml of distilled water. The vial was allowed to stand for 30minutes, and it was swirled occasionally. A calibration graph for the estimation of the different concentration of immunoglobulin levels is formed by plotting concentration (deduced by the multiplication of the standard factor of each immunoglobulin reagent by the standard concentration of each reagent) against absorbance (produced from 6 constituted samples made up of the standard reagent diluted with Nacl in different ratios). Then 0.5ul of serum samples were pipetted into appropriately labeled test tubes and 350ul of the IgA, and IgG reagent. For IgM testing, 0.5ul of blood serum was pipetted into a test tube and 500ul of the reagent are mixed. Each mixture in separate test tube is pre-warmed for 2minutes. Then the well calibrated analyzer with each individual reagent specifications is used for production of absorbance that its corresponding absorbance was read with the use of the appropriate calibration formed for each reagent. Diluents bottles were filled with distilled water. Assay procedure was programmed on fully smart chemistry analyzer using multi-standard programmes method. Test identification was entered for IgA, IgG and IgM. Assay conditions were set accordingly: 340nm for IgM and IgA, and 540nm for IgG, 37°C temperature, cuvette light path of 1.0cm and absorbance range of 0-0.25A. Calibrations were made at manufacturer’s specifications for Automated Chemistry Analyzer. Reference Range of Immunoglobulins in adults: IgG-700-1600 mg/dL, IgA-70-400mg/dL, IgM-40-230mg/dl. Reference Range of Immunoglobulins in infants: IgG-231-1411mg/dL, IgA-0-83mg/dL, IgM-0-145mg/dL [27].
3. Results
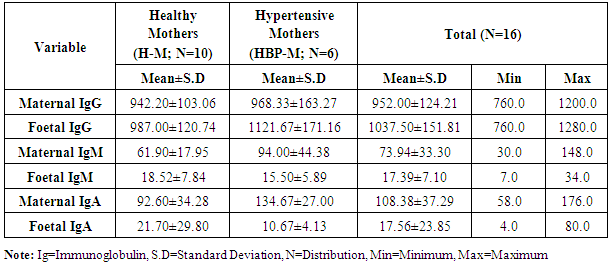
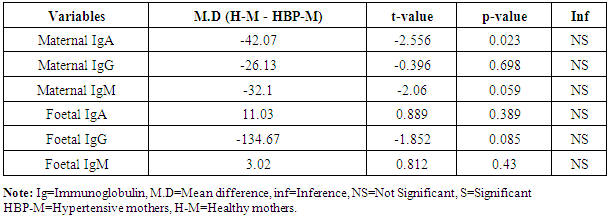
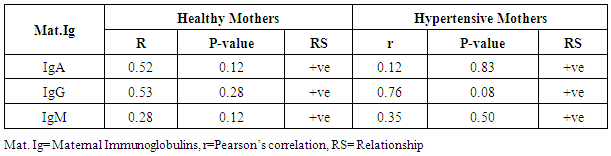
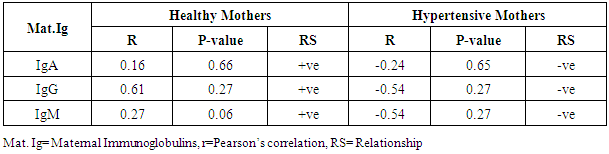
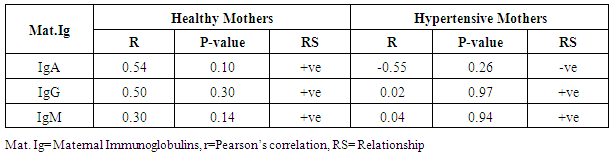
- The statistical analyses from the Immunoglobulin (A, G and M) assay were presented in Tables 1-5. The comparison of mean difference in measured parameters of healthy mothers (H-M) and hypertensive mothers (HBP-M) were presented in Tables 1 and 2. Table 3 presents the relationship between maternal IgA, IgG, IgM and Foetal IgA and table 4 shows the relationship between maternal IgA, IgG, IgM and Foetal IgG. Table 5 presents the relationship between maternal IgA, IgG, IgM and Foetal IgM.
|
|
|
|
|
4. Discussion
- The mean maternal IgG levels of mothers with hypertension and healthy state were 968.33±163.27 mg/dl and 942.20±103.06 mg/dl respectively, while their corresponding mean foetal IgG values were 1121.67±171.16 mg/dl and 987.00±120.74 mg/dl. Also, the mean maternal IgM levels of mothers with hypertension and healthy state were 94.00±44.38 mg/dl and 61.90±17.95 mg/dl respectively, while their corresponding mean foetal IgM levels were 15.50±5.89 mg/dl and 18.52±7.84 mg/dl respectively. The mean maternal IgA levels of mothers with hypertension and healthy state were 134.67±27.00 mg/dl and 92.60±34.28 mg/dl respectively, while their corresponding mean foetal IgA levels were 10.67±4.13 mg/dl and 21.70±29.80 mg/dl respectively. Our study shows a significant difference in the IgG levels of hypertensive and healthy mothers. The foetal IgG levels were markedly high in hypertensives than in healthy subjects. The mean maternal IgG levels in pregnant hypertensive subjects were 968.33±163.27 mg/dl compared to 942.20±103.06 mg/dl in pregnant healthy subjects. This agrees with the study by Ahsan et al who documented higher levels of IgG in pregnancy-induced hypertension. They reported that IgG concentration was higher in pre-eclampsia while normotensive mothers had lower concentration of IgG and IgM. In this present study, we could see lower levels of IgG and IgM in healthy mothers who were normotensive. However these findings appear to be at variance with Amah-Tariah and associates who reported that healthy pregnant had significantly higher IgG and lower levels of IgM, compared to subjects with pre-eclampsia and gestational diabetes. Infact they mentioned that subjects with pre-eclampsia showed far lower levels of IgG. Though our study was not precise on subjects with pre eclampsia, the fact that it is as well a hypertensive-related condition in pregnancy, we do not expect any much difference considering hypertension generally. Hence finding higher levels of IgG in our hypertensive patients is a forerunner to what could be expected in pre eclampsia and even eclampsia. This however was confirmed to us from the reports of Ahsan and associates. Our maternal IgG levels were lower than the foetal IgG levels. This also is at variance with the study by Salimonu et al, who documented higher maternal IgG levels. They also reported that the mean foetal IgA and IgM levels were lower than the foetal IgG. This is in firm consent with our study where the foetal IgG was significantly higher than the others. Moreover our study was able to show variations in foetal Immunoglobulins in both hypertensive and healthy mothers. Here mean foetal IgG in hypertensive and healthy mothers were 1121.67±171.16 mg/dl and 987.00±120.74 mg/dl respectively. The hypertensive subjects also showed higher levels of foetal IgG. This may not be medically unproven as it is established from previous studies of Saji and Malek together with their counterparts that IgG transfer from mother to foetus is highest during the third trimester of pregnancy. Hence finding more IgG in hypertensive mothers could explain how much could have been transferred to the foetus as well. It is no doubt therefore that the amount of immunoglobulins transferred depends on the amount of total IgG in the maternal sera which will determine the level of immunoglobulin transferred to the foetus (Hartter et al, 2000; Okoko et al, 2001; Englund et al, 2007). The levels of the mean foetal IgA and IgM were significantly low compared to foetal IgG. This is in agreement with the findings of Salimonu and associates. The variations between foetal IgG and IgM were not significant, though their levels were higher in healthy mothers.The mean IgA and IgM levels in hypertensive mothers were higher than those in healthy mothers in this present study. This is in agreement with the study by Amah-Tariah et al who documented that IgM levels were lower in healthy mothers compared to those with pre-eclampsia and gestational diabetes. They also observed in their study that healthy pregnant mothers had a gradual decrease in all levels of three immunoglobulins from 1st to 3rd trimester of pregnancy. This also agrees with our present study in part, as we have observed that the three immunoglobulins; IgG, IgM and IgA has lower levels in the healthy subjects compared to their hypertensive counterparts. We could not however establish if these values declined with gestational age. Amah-Tariah and associates also reported from their research that IgA levels were lowest in healthy mothers when compared to mothers with malaria and HIV. Although our study revealed that the mean values of IgM were lowest in healthy mothers compared to hypertensive mothers.This present study and previous research work was able to establish that in healthy pregnant subjects, the concentration of immunoglobulins in the blood declines with gestational age. It is thus worthy of note that decrease in immunoglobulin levels as noticed in healthy mothers is protective for the foetus, consenting with theories about suppression of maternal immunity during pregnancy in order to prevent rejection of the foetal allograft.
5. Conclusions
- Based on the facts established from this study and that of previous work, it is true that the deleterious effects of hypertension on pregnancy are due to imbalances in the level of maternal immunoglobulins. It is therefore imperative to conclude that the raised immunoglobulin levels in hypertensive pregnant as seen in our present research could as well explain the possibilities for foetal demise and other complications. Hence good blood pressure control as well as the possibility of achieving decline in the raised levels of immunoglobulin could be the salvage for the growing foetus. More so for the fact that in most pregnancies complicated by hypertensive disorders, reduction in blood pressure did not attain the expected outcome for the foetus. This study has further widened the scope for more research in the area of serum immunoglobulins. It has also brightened the interest into research areas in the subject and specialty of immunology.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML