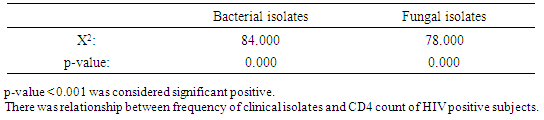Evelyn Ukamaka Urama, Ifeoma Bessie Enweani, Ifeanyi Onyema Oshim, Benedictta Chinweoke Okeke-Nwolisa, Godsplan Uchechukwu John
Department of Medical Laboratory Science, Faculty of Health Sciences and Technology, Nnamdi Azikiwe University, Awka, Nigeria
Correspondence to: Evelyn Ukamaka Urama, Department of Medical Laboratory Science, Faculty of Health Sciences and Technology, Nnamdi Azikiwe University, Awka, Nigeria.
| Email: |  |
Copyright © 2018 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
The upper respiratory tract infections are among the first few secondary infections commonly seen in Human Immunodeficiency Virus (HIV) infected patients. Hence, this study was undertaken to document the occurrence of bacterial and fungal respiratory tract infections in HIV infected patients and correlate the levels of CD4+ count of the HIV positive subjects. Total number of 208 subjects were recruited for this study which included 135 test subjects and 73 control subjects. Ethical approval was obtained from Nnamdi Azikiwe University Teaching Hospital Nnewi. Pre-tested, interviewer administered questionnaires and laboratory test results were used to collect data, which were analyzed using Statistical Package for Social Sciences, version 20. Chi-square and correlation set at 95% confidence limit and 0.001% level of significance. From 135 test subjects, 73(54.07%) clinical bacterial isolates were obtained. Five different bacterial species were isolated which included Klebsiella pneumoniae being predominant (23%), followed by Staphylococcus aureus (21%), Streptococcus pneumonia (13%), Pseudomonas (9%) and Escherichia coli (3%). The frequency of clinical bacterial isolates and HIV positive subjects were statistically significant χ2 at=30.483a, p=0.000. Out of 135 test subjects, 67(49.6%) clinical fungal isolates were recovered. Eight different clinical fungal isolates were recovered which included Candida albicans (21%) which was the predominant species, followed by Aspergillus fumigatus (11%), Penicillum marneffei (10%), Candida krusei (6%), Aspergillus niger (3%), Candida tropicalis (2%), Aspergillus flavus (2%), and Phialemonium curvatum (2%). The frequency of clinical fungal isolates and HIV positive subjects were statistically significant χ2 at =19.678a, p=0.000. There exist relationship between the prevalence of clinical isolates and HIV –sero positive subject at p<0.001. This showed that the frequency of clinical isolates and HIV-sero positive subjects were statistically significant. However, its occurrence is HIV disease condition and low CD4 count dependent. This was attributed to depressed immunity caused by the presence of HIV infection which continually depletes the CD4 leaving the body prone to these opportunistic infections.
Keywords:
Upper respiratory tract infections, HIV, CD4 Count
Cite this paper: Evelyn Ukamaka Urama, Ifeoma Bessie Enweani, Ifeanyi Onyema Oshim, Benedictta Chinweoke Okeke-Nwolisa, Godsplan Uchechukwu John, Microbiological Profile of Respiratory Tract Infections among HIV Sero-positive Subjects Attending Nnamdi Azikiwe University Teaching Hospital Nnewi, Nigeria, American Journal of Medicine and Medical Sciences, Vol. 8 No. 3, 2018, pp. 37-42. doi: 10.5923/j.ajmms.20180803.01.
1. Introduction
Acquired Immunodeficiency Syndrome (AIDS) is a disease of human immune system caused by infection with Human Immunodeficiency Virus (HIV) [1]. About 53% [39–65%] of people living with HIV were receiving antiretroviral treatment in 2016 while 21 million people were receiving antiretroviral treatment by mid-2017 [2]. There were approximately 36.7 million people worldwide living with HIV/AIDS [3]. Approximately 160,000 people died from AIDS-related illnesses in Nigeria in 2016 [4]. Although, HIV prevalence among adults is remarkably small (2.9%) compared to other sub-Saharan African countries such as South Africa (18.9%) and Zambia (12.4%), the size of Nigeria's population means 3.2 million people were living with HIV in 2016 [5].Respiratory tract infection refers to any of a number of infectious diseases involving the respiratory tract. An infection of this type is normally further classified as an upper respiratory tract infection (URI or URTI) or a lower respiratory tract infection (LRI or LRTI). Lower respiratory infections, such as pneumonia, tend to be far more serious conditions than upper respiratory infections, such as the common cold [6]. Upper respiratory tract infections (URI or URTI) are the illnesses caused by an acute infection which involves the upper respiratory tract: nose, sinuses, pharynx or larynx. This commonly includes: tonsillitis, pharyngitis, laryngitis, sinusitis, otitis media, and the common cold [6].Streptococcus pneumoniaeStreptococcus pneumoniae, or pneumococcus, is a Gram-positive, alpha-hemolytic, aerotolerant, aerobic member of the genus Streptococcus [7]. Streptococcus pneumoniae resides asymptomatically in the nasopharynx of healthy carriers. However, in susceptible individuals, such as elderly and immunocompromised people and children, the bacterium may become pathogenic, spread to other locations and cause disease. Streptococcus pneumoniae is the main cause of community acquired pneumonia and meningitis in children and the elderly, and of septicemia in HIV-infected persons.Staphylococcus aureus is the most common species of staphylococcus to cause Staphylococcus infections and is a successful pathogen due to a combination of nasal carriage and bacterial immuno-evasive strategies [8]. It is still one of the five most common causes of nosocomial infections and is often the cause of postsurgical wound infections.Klebsiella pneumoniae is a Gram-negative, non-motile, encapsulated, lactose-fermenting, facultative anaerobic, rod-shaped bacterium. Although found in the normal flora of the mouth, skin, and intestines [8]. It can cause destructive changes to human lungs if aspirated, specifically to the alveoli resulting in bloody sputum. In the clinical setting, it is the most significant member of the Klebsiella genus of Enterobacteriaceae. Klebsiella oxytoca and Klebsiella rhinoscleromatis have also been demonstrated in human clinical specimens.Candida is a genus of yeasts and is the most common cause of fungal infections worldwide [9]. In debilitated or immunocompromised patients, or if introduced intravenously, candidiasis may become a systemic disease producing abscess, thrombophlebitis, endocarditis, or infections of the eyes or other organs [10].Aspergillus is a member of the deuteromycetes fungi, which is a group with no known sexual state. With DNA evidence forthcoming, it is likely that all members of the genus Aspergillus are closely related and should be considered members of the Ascomycota. Members of the genus possess the ability to grow where there is a high osmotic concentration (high sugar, salt, etc.) [11]. The most common pathogenic species are Aspergillus fumigatus and Aspergillus flavus, which produces aflatoxin which is both a toxin and a carcinogen, and which can contaminate foods such as nuts. The most common species causing allergic disease are Aspergillus fumigatus and Aspergillus clavatus [12]. Aspergillosis is the group of diseases caused by Aspergillus. The most common subtype among paranasal sinus infections associated with aspergillosis is Aspergillus fumigatus [13]. Usually, only patients with already weakened immune systems or who suffer other lung conditions are susceptible [14]. Penicillum marneffi, a dimorphic fungus endemic to southeast especially Northern, Thailand and Vietnam Asia and Southern part of China [15]. This causes penincillosis. The prevalence of infection has increased substantially especially in person with HIV. Persons affected by Penicillosis usually have AIDS with low CD4 lymphocytes count of 100cells/µl with average cells of 63.5. The infection is associated with high mortality rate if timely treatment with appropriate antifungal drugs is not administered [16]. In Northern Thailand Penicillosis is now the third most common opportunistic infection after tuberculosis and Cryptococcosis. Penicillosis is acquired by inhalation of micro conidia from the mycelia phase of the organism. Reactivation of a silent focus of infection that was acquired years earlier can occur when cellular immunity wanes and it is the presumed mechanism for disease occurrence in non-endemic areas. It exists in seasonality with increase in rainy months [17]. The increase in the frequency of clinical isolates seen on CD4 cells less than 200 cell confirmed that the frequency increases with a decrease in CD4 and vice versa. This was attributed to depressed immunity caused by the presence of HIV infection which continually depletes the CD4 leaving the body prone to opportunistic infections.
2. Materials and Methods
Sample population and sampling techniqueThe subjects for this study comprises of 75 HIV positive subjects on ART, 60 HIV patients not on ART attending Out Patient Clinic at NAUTH, and 73 non HIV subjects from all forms of life. These subjects were randomly selected.Sample sizeThe sample size was derived using the formular.N = Z2 X P (1-P) /d2 [18].Where N = Minimum sample size.P = Prevalence rate of HIV patients = 0.034 (3.4%) (NACA, 2013). National HIV prevalence rate was used.D = Desired level of significance = 0.001 (1%)Z = Confidence interval = 1.96 (95% confidence Interval)N = (1.962) X 0.034(1-0.034)/0.052N = 50.47.Inclusion criteriaThe study included HIV positive subjects on antiretroviral therapy, HIV subjects that are not on antiretroviral therapy and non HIV subject from all works of life serving as control.Exclusion criteriaHIV positive subjects and HIV-sero negative subjects who were pregnant and also on antimicrobial treatment.Ethical approvalEthical clearance was sought for and obtained from Nnamdi Azikiwe University Teaching Hospital Nnewi Ethics Committee in accordance with the code of ethics for biological research involving human subjects. Individual consent was also obtained from the patients before sample collection. (the approval number: NAUTH/ CS/ 66/ VOL.6/ 97). The study design in December, 2016.Collection of sputum:A wide mouthed srew-capped, sterile labeled container was given to each HIV positive. They were asked to expectorate sputum into the containers.Collection of blood:About 4ml of blood was collected from each participant in EDTA container for HIV screening and CD4+ T cell count. All aseptic conditions were observed accordingly according to the methods described by [19].Processing of sputum samplesEach sputum sample was inoculated on two sets of Sabourand’s dextrose agar (SDA) impregnated with antibiotic, chloramphenicols and SDA without antibiotic. Media plates were incubated at 25°C for two weeks with examinations for growth on every three days. Along with this the same sputum samples were inoculated on two sets of MacCkonkey agar and Blood agar plates impregnated with antifungal (fluconazole) and without antifungal. Media plates were incubated at 37°C for 24hrs respectively. Media plates with growth were identified macroscopically and microscopically. A criterion for positive growth was based on two plates from the same patients yielding the same growth [20].Identification of bacterial and fungal isolatesThe different bacterial and fungal isolates were identified macroscopically and microscopically. Also biochemical tests and cultural technique were adopted as described by [20].HIV screeningHIV screening was done to determine the HIV status of the control subjects using Determine HIV-1/2 test kit, Uni-Gold kit (Trinity Biotech) and STAT-PAK (Chembio Diagnostic System, Inc.) The test group were known HIV sero-positive subjects attending out- patient clinic at Nnamdi Azikiwe University Teaching Hospital Nnewi.CD4+ t- cell countThe control subjects were screened to determine their HIV status using immunochromatographic technique. Flow cytometry method was used for CD4+ count. The samples were run using cyflow count machine (Partec Germany). Results were expressed as number of cells/µl. Reference range for CD4+ were taken as 600-1200cells/µl.Cyflow counter for automated CD4+ T cell count.Procedure20µl of EDTA whole blood will be collected into partec test tube (Rohren tube). The 20µl of CD4+ T cell antibody will be added into the tube. The content was mixed and incubated in the dark for 15minutes at room temperature. 800µl of CD4+ buffer was added into the mixture and mix gently. The partec tube will be plugged on cyflow counter and the CD4+ t cells will be displayed as peak and interpreted as figures.
3. Results
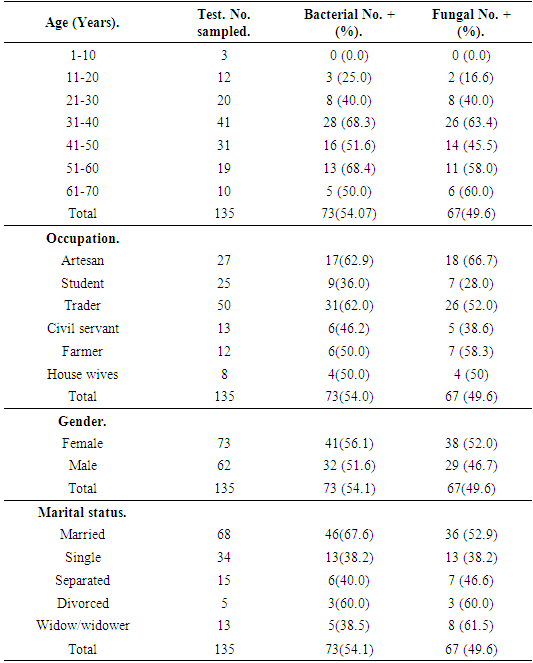
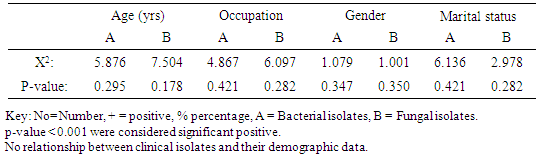
Result of the demographic information of the subjects revealed that association of bacterial isolates in relation to age range indicated that bacterial isolates had highest frequency on age range of 51-60 with 13(68.7%) bacterial isolates, followed by age range of 31-40 with 28(68.3%) isolates and least bacterial isolates was recorded against age range 1-10 without any 0(0.00) bacterial isolate. X2=5.876a, p=0.295 (Table 1). Association of fungal isolates in relation to their ages had highest frequency on age range of 31-40 with 26(63.4%) fungal isolates, followed by age range of 61-70 with 6(60.0%) fungal isolates and least fungal isolates was recorded against age range 1-10 without any 0(0.00) fungal isolate. X2=7.5049a, p=0.175 (Table 1).Table 1. Frequency of clinical isolates according to demographic data of the subjects
 |
| |
|
Association of bacterial isolates to occupation showed that Artesan recorded highest frequency of bacterial isolates with 17(62.9%) bacterial isolates, followed by traders with frequency of 31 (62.0%) bacterial isolates, the least bacterial isolates was recorded on house wives with 9(36.0%) bacterial isolates. X2=4.867a, p=0.421(Table 1). Association of fungal isolates in relation to occupation also recorded highest frequency on artisan with 18(66.7%) fungal isolates, followed by traders with frequency of 26 (52.0%) fungal isolates, the least frequency of fungal isolates was recorded on students with 7(28.0%) fungal isolates, X2=6.097a, p=0.282 (Table 1).Association of frequency of clinical isolates in relation to Gender recorded that female had highest frequency of bacterial isolates with 41 (56.1%) bacterial isolates, followed by male with 32(51.6%) bacterial isolates. X2=1.079a, p=0.347 (Table 1). Association of fungal isolates also had highest frequency of fungal isolates on female with 38(52.0%) fungal isolates, followed by the male with frequency of 29(46.7%) fungal isolates. X2=1.001, p=0.282 (Table 1).Association of clinical isolates in relation to marital status indicated that married subjects recorded highest frequency of bacterial isolates with 46(67.6%) bacterial isolates, followed by divorced subjects with 3(60.0) bacterial isolates, least frequency of clinical bacterial isolates was recorded on singles with 13(38.2%) isolates. X2=8.904, p=0.052 (Table 1). Widow and widower had highest frequency of clinical isolates with 8 (61.5%) fungal isolates, followed by Housewives with 3(60.0%) fungal isolates and Singles had the least frequency of fungal isolates with 13(38.0%) fungal isolates. X2=19.678a, p=0,052 (Table 1). Relationship between sociodemographic characteristics and clinical isolates was showed in (Table 2).Table 2. Relationship between socio-demographic characteristics and Clinical isolates from HIV positive subjects
 |
| |
|
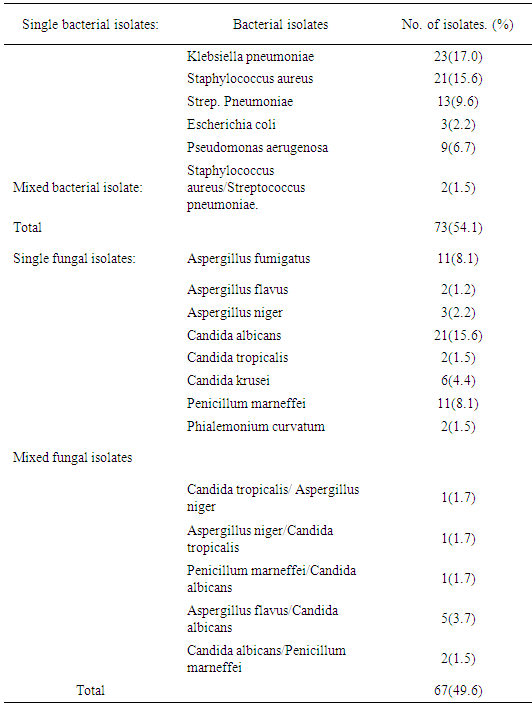
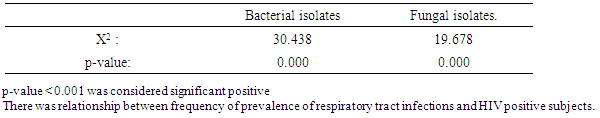
Result of demographic data indicated that, 208 subject was recruited for this study which included 135 HIV sero-positive subjects and 73 HIV sero-negative subjects, 73(54.1%) sputum samples. Out of 135 HIV positive subjects, five bacterial species which occurred as single and mixed growth were isolated which included Klebsiella pneumoniae, Staphylococcus aureus, Streptococcus pneumoniae, Pseudomonas aerugenosa and Escherichia coli. klebsiella pneumoniae dominated other species with frequency of 23(17.0%) bacterial isolates, followed by Staphyjococcus aureus with 21(15.6%) bacterial isolates and least frequency of bacterial isolates was recorded on scherichia coli with 3(2.2%) bacterial isolates. X2=30.438a, and p =0.000. Results from 135 HIV positive subjects sampled showed that, eight fungal species which occurred as single and mixed growth were isolated which included Candida albicans with 21(15.0%) fungal isolates and was predominant, Candida tropicalis, Candida krusei, Aspergillus niger, Aspergillus fumigates, Penicillum marneffei and Phialemonium curvatum were all isolated. X2=19.678a, p=.0.000 as was shown in (Table 3). Relationship between prevalence of respiratory tract infections and HIV Sero positive subjects was shown in (Table 4). Table 3. Prevalence of respiratory tract infections from HIV Sero- positive subjects
 |
| |
|
Table 4. Relationship between prevalence of respiratory tract infections and HIV Sero positive subjects
 |
| |
|
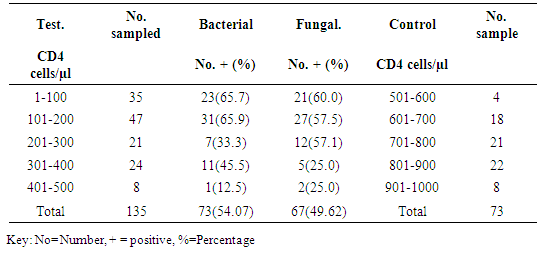
Association of bacterial isolates with CD4 Count recorded highest bacterial isolates on CD4 range of 101-200 with 31(65.9) bacterial isolates, followed by CD4 range of 1-100 with 23(65.7%) bacterial isolates and CD4 range of 400-500 recorded the least number of bacterial isolates 8(12.5%). X2=84.000a, p=0.000. Fungal isolates recorded highest frequency on CD4 count ranges of 101-200 with 27(40.2%) fungal isolates, followed by CD4 range of 1-100 with 21(13.3%) fungal isolates and the least fungal isolates was recorded against CD4 ranges of 401-500 with 2(2.9%) fungal isolates. X2=78.000a, p=0.000 as shown in (Table 5). Relationship between frequency of Clinical isolates and CD4 count of the subjects was shown in (Table 6).Table 5. Association of bacterial and fungal isolates according to CD4
 |
| |
|
Table 6. Relationship between frequency of Clinical isolates and CD4 count of the subjects
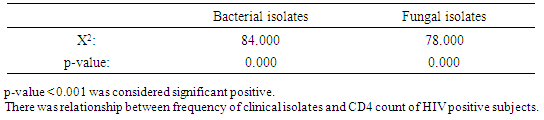 |
| |
|
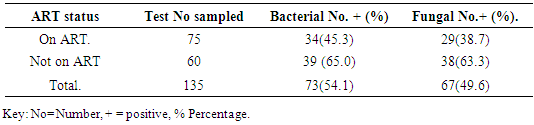
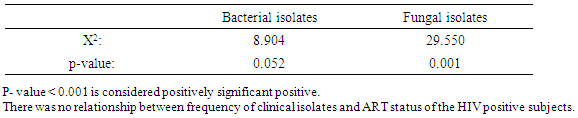
Association of clinical isolates in relation to ART status showed that bacterial isolates had highest frequency on subjects not on ART with 39(65.0%) bacterial isolates and least bacterial isolates was recorded on subjects on ART with 34(45.3%) bacterial isolates. X2=8.904a, p=0.052. Fungal isolates had highest frequency on subjects not on ART with 38(63.3%) and on subjects on ART with 34(38.7%) isolates. X2=29.550a, p=0.001 as was shown in (Table 7). Relationship between frequency of Clinical isolates and ART status was shown in (Table 8).Table 7. Association of the frequency of clinical isolates according to ART status
 |
| |
|
Table 8. Relationship between frequency of Clinical isolates and ART status
 |
| |
|
4. Discussion
Infection with Human immunodeficiency virus is the most powerful known risk factor predisposing opportunistic infections. There was no statistical relationship (p>0.001) between frequency of clinical isolates and any of the following factors-Age, Gender, Occupation and Marital status of the subjects. This also mean that clinical isolates are not Age, Gender, Occupation or Marital status dependent. Klebsiella pneumoniae with frequency of 23(17.0%) bacterial isolates dominated other bacterial isolates. Klebsiella pneumoniae is a normal flora of the mouth and other sites but can cause destructive changes to human lungs when aspirated and also causes infection due to compromised immunity [7]. This study agrees with similar study by [21]. Candida albicans with frequency of 21(15.6%) fungal isolates dominated other fungal isolates. Candida albicans is one of the candida specie that is a normal flora of the body. Where it causes infection, it is of prognostic value as its presence indicates progression of immunity [22]. Their exist relationship between the frequency of clinical isolates and HIV positive subject at p<0.001. This means that the frequency of clinical isolates and HIV positive subjects were statistically significant positive or that its occurrence is HIV disease dependent. This shows that the frequency of clinical isolates was dependent on the category of people sampled. These are known HIV positive subjects; the increase frequency of the clinical isolates was attributed to their depressed immunity. The increase in the frequency of clinical isolates seen on CD4 cells less than 200 cell confirmed that the frequency increases with a decrease in CD4 and vice versa. This was attributed to depressed immunity caused by the presence of HIV infection which continually depletes the CD4 leaving the body prone to opportunistic infection. This is synonymous to a similar work done by [21] that showed relationship at p<0.001 between frequency of clinical isolates and CD4 count. This means that frequency of isolates was CD4 count dependent. The ART naïve subjects had higher frequency of clinical bacterial isolates with 39(65.0%) bacterial isolates when compared to subjects on ART with 34(45.3%) bacterial isolates. There was no positive statistical significant relationship at between frequency of clinical bacterial isolates and ART therapy since p = 0.052. The ART naïve subjects had higher frequency of clinical fungal isolates with 38(63.3%) fungal isolates when compared to subjects on ART with 29(38.7%) fungal isolates. There was no positive statistical significant relationship at between frequency of clinical isolates and ART therapy since p=0.001. Attitudinal behavior of the subjects not taking their regimen as required and not living up to dietary requirement which helps the regimen to give its best results are limiting factors [23]. This was synonymous to similar work done by [23] whom reported that most people are placed on ART for a very long time but still maintain low CD4 and increased frequency of isolates.
5. Conclusions
Upper respiratory tract infections are among the first few secondary infections commonly seen in Human Immunodeficiency Virus (HIV) infected patients and infection with Human immunodeficiency virus is the most powerful known risk factor predisposing these opportunistic infections whenever there is drastically decreased of CD4 count in seropostive HIV patients. However, the present study shown the occurrence of bacterial and fungal respiratory tract infections in HIV infected patients and correlate the levels of CD4+ count of the HIV positive subjects with clinical bacterial and fungal infections. This shows that the frequency of clinical isolates was dependent on the category of subjects sampled. These are known HIV positive subjects; the increase frequency of the clinical isolates was attributed to their depressed immunity. The increase in the frequency of clinical isolates seen on CD4 cells less than 200 cell which confirms that the frequency increases with a decrease in CD4 and also, continually depletes the CD4 count could leave the body prone to these opportunistic infections.
References
| [1] | Sepkowitz, K. A. (2001). Aids –the first 20 years. Natina l English Journal medicine. 344(23): 1764- 1772. |
| [2] | World Health Organization (2017). Global Data and Statistcs of HIV and AIDS. |
| [3] | UNAIDS (2017). Global Report on HIV/AIDS Epidemic last updated. |
| [4] | UNAIDS (2017). Data Book. |
| [5] | UNAIDS (2017). AIDS info. Accessed. |
| [6] | Eccles, M.P., Grimshaw, J.M. and Johnston M. (2007). Applying psychological theories to practice: identifying factors predictive of managing upper implementation Science Respiratory tractinfections without antibiotics evidence-based clinical 2: 26. |
| [7] | Reyan, K. J.and Ray, C. G. (2004). Sherris Medical Microbiology (4th Ed.). McGraw. Hill ISBN 0-8385-8529-9. |
| [8] | Cole, A. M., Tahk, S., Oren, A., Yoshioka, D., Kim, Y. H., Park, A. and Ganz, T. (2001). Determinants of Staphylococcus aureus nasal carriage. Clinical Diagnostic Laboratory Immunology. 8 (6): 1064–1069. |
| [9] | Manolakaki, D., Velmahos, G., Kourkoumpetis, T., Chang, Y., Alam, H. B., De Moya, and Mylonakis, E. (2010). Candida infection and colonization among trauma patients. Virulence, 1(5), 367- 375. |
| [10] | Enfert, C and Hube, B (editors) (2007). Candida: Comparative and Functional. Genomics Caister Academic Press. ISBN 978-1-904455-13-4. |
| [11] | Bennett, J.W. (2010). An overview of the genius Aspergillus. Aspergillus; Molecular biology and genomics: Caister Academic press: ISBN 978-1-904455-53-0. |
| [12] | Geiser, D. (2009). Sexual structure in aspergillus structure, morphology, importance and genomics. Medical mycology. Official publication the international society for human and animal mycology.47.suppl (51)521- 526.pmd18608901 edit. |
| [13] | Bozkurt, M.K, Ozcelik, T, Saydan, L. and Kutluay, L. (2008). A case of isolated aspergillosis of Caister Academic Press. ISBN 978-1-904455-13-4. |
| [14] | Wilson, W.R (2001). Current diagnosis and treatment of infectious diseases. Lange. (2001). |
| [15] | Duong, T.A. (1996). Infection due to Penicillum marneffei an emerging pathogen review of 155 reported cases. Clinical infection disease 23:125-130. |
| [16] | Supparatpinyo, K., Khamwan, C., Baosoung, V., Nelson, K. and Sirisanthana, T.. (1994). Disseminated Penicillum marneffei infection in Southeast Asia. Lancet 344:110-113. |
| [17] | Chariyalertsak, S., Sirisanthana, T., Supparatpinyo, K. and Nelson, K. (1996). Seasonal variation of disseminated Penicillum marneffei infection in northern Thailand a clue to reservoir. Journal of infectious disease 13 (6):1490-1493. |
| [18] | Naing, l., Winn, O. and Rush, B. N. (2008). Practical issues for calculating sample size for prevalence studies. Archieves of orofacial Sciences (1):9-14. |
| [19] | Cheesbrough, M. (2006). Summary of the clinical and laboratory features of microorganisms. District Laboratory Practice in Tropical Countires, Part 2, and. 2nd edition Cambridge University press, New York, USA. Pp 178-298. |
| [20] | Ochei, J. and Kolkhatkar, A. (2007). Medical laboratory Science. Theory and. Practice Department of microbiology, College of Medicine, Sultan Qaboos University, Muscat. Tata McGraw-Hill Publishing Company Limited, New Delhi. |
| [21] | Bharathi, B., Sivasankar, S. and Swamidoss, D. (2010). Incidence of bacterial and fungal co-infection in some HIV infected Indian population. Indian Journal of Biotechnology. 3 (2): 199. |
| [22] | World Health Organization (2011). Global HIV/AIDS response. Epidemic update and health sector progress towards universal access. WHO, Geneva Switzerland. |
| [23] | Obi, C.L., Onabolu, B., Momba, M.N.B., Igumbo, J.J., Ramalivahna, J. and Blessing, P.O. (2006). The interesting cross path of HIV/AIDS and water in southern Africa in special references to south African water. South Africa. 32: 323-324. |



 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML