-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2017; 7(2): 400-404
doi:10.5923/j.ajmms.20170712.03

Male Body Mass Index and Intracytoplasmic Sperm Injection Outcomes in Men with Non-Obstructive Azoospermia
Ahmed F. El-Sherbiny1, Eman A. Hassan2, Mohamed Shehata3, Mokhtar M. Elsapagh4
1Venereology and Andrology Department, International Islamic Center for Population Studies & Researches, Al-Azhar University, Cairo, Egypt
2Embryology Department, International Islamic Center for Population Studies and Research (IICPSR), Al-Azhar University, Cairo, Egypt
3Obstetrics and Gynecology Department, International Islamic Center for Population Studies and Research (IICPSR), Al-Azhar University, Cairo, Egypt
4Venereology and Andrology Department, Faculty of Medicine, Tanta University, Egypt
Correspondence to: Eman A. Hassan, Embryology Department, International Islamic Center for Population Studies and Research (IICPSR), Al-Azhar University, Cairo, Egypt.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

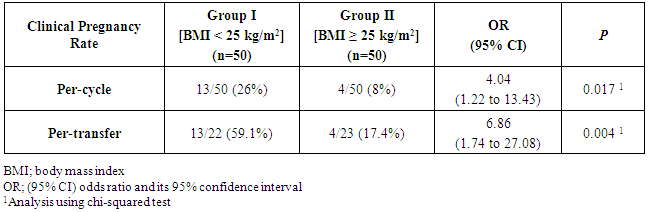
The aim of the current study was to evaluate the relationship between male body mass index (BMI) and the intracytoplasmic injection (ICSI) outcomes in men undergoing testicular sperm extraction (TESE) and intracytoplasmic sperm injection (ICSI) for non-obstructive azoospermia (NOA). Methods: Prospective comparative study was conducted at the Assisted Reproduction Unit, International Islamic Center for Population Studies and Researches, Al-Azhar University. All included patients were divided into two groups; group I, including patients with a BMI < 25 kg/m2; and group II, including patients with a BMI ≥ 25 kg/m2 (Fifty patients planned to undergo TESE and ICSI for NOA were included in each group). The primary outcome was clinical pregnancy rate. Secondary outcomes included fertilization rate, embryo development and embryo grade. Results: The rate of positive sperm retrieval was comparable in both groups. The mean values of fertilization rates and embryo development rates were significantly higher in patients of group I. also rate of grade A embryo was significantly higher in patients of group I. The per-cycle and per-transfer clinical pregnancy rates were both significantly higher in patients of group I [13/50 (26%) vs. 4/50 (8%), respectively, OR 4.04, 95% CI (1.22 to 13.43), p=0.017; and 13/22 (59.1%) vs. 4/23 (17.4%), respectively, OR 6.86, 95% CI (1.74 to 27.08), p=0.004; respectively]. Conclusion: Among men with NOA, obesity seems to be associated with reduced ICSI outcomes in terms of reduced fertilization, embryo development and clinical pregnancy rates.
Keywords: Azoospermia, Non-obstructive Azoospermia, Intracytoplasmic sperm injection, Body mass index
Cite this paper: Ahmed F. El-Sherbiny, Eman A. Hassan, Mohamed Shehata, Mokhtar M. Elsapagh, Male Body Mass Index and Intracytoplasmic Sperm Injection Outcomes in Men with Non-Obstructive Azoospermia, American Journal of Medicine and Medical Sciences, Vol. 7 No. 2, 2017, pp. 400-404. doi: 10.5923/j.ajmms.20170712.03.
1. Introduction
- Azoospermia is defined as the absence of spermatozoa in the ejaculate after assessment of centrifuged semen sample on at least two occasions. The incidence of azoospermia is 1% in general population and 10-15% of infertile male partners [1]. Two broad categories are defined: obstructive azoospermia (OA) and non-obstructive azoospermia (NOA). The latter is defined when azoospermia is due to minimal or no production of fully developed spermatozoa in the testicles [2]. The standard management of infertile men with NOA is testicular sperm extraction (TESE) in conjunction with intracytoplasmic sperm injection (ICSI) [3]. Proposed factors which might influence the outcome of sperm recovery include age, underlying etiology, testicular size and serum follicle stimulating hormone (FSH) level, and histological testicular pattern [4]. Obesity is a prevalent disease in modern life. It is a major component of the metabolic syndrome, and has been associated with metabolic, endocrine and hormonal changes [5]. The association between female obesity and reduced fertility and assisted reproduction outcomes is well-established [6]. Similar association with male obesity is not that well-established, however, the results of studies remain conflicting [7, 8]. The aim of the current study was to evaluate the relationship between male obesity represented by body mass index (BMI), and the ICSI outcomes in men undergoing TESE and ICSI for NOA.
2. Methods
- The current study was conducted at the Assisted Reproduction Unit, International Islamic Center for Population Studies and Researches, Al-Azhar University; during the period between January 2015 and December 2015. The study protocol was in agreement to the Helsinki Declaration for the Principles of Ethical Medical Research [last updated in Brazil, 2013]. The study had been approved by the Ethical Research Committee at the Dermatology, Venereology and Andrology Department, Faculty of Medicine, Al-Azhar University. All participating patients signed informed written consent after thorough explanation of the purpose and procedure of the study. The study included 100 male patients with a diagnosis of non-obstructive azoospermia, who were planned to undergo testicular sperm extraction (TESE) as part of intracytoplasmic sperm injection (ICSI). Patients with aspermia, oligozoospermia, Klinefelter syndrome, or history of cryptorchidism, patients maintained on hormonal therapy, patients with testicular volume < 10 ml, and whose female partners were > 35 years old or have diminished ovarian reserve were not included in the study.Azoospermia is defined as complete absence of sperms in the ejaculated semen specimen. Azoospermia was confirmed by the analysis of at least two different centrifuged, ejaculated specimens, according to the WHO guidelines [9].All included patients were divided into two groups: group I, including patients with a body mass index (BMI) < 25 kg/m2; and group II, including patients with a BMI ≥ 25 kg/m2. BMI was calculated as the weight (in kilograms) divided by the squared height (in squared meters) [10]. All included patients underwent TESE on day of oocyte retrieval. All included couples who had positive sperm retrieval were subjected to ICSI.Ovarian stimulation was carried out by a combination of gonadotrophin releasing hormone agonist and human menopausal gonadotrophin. Ovulation is triggered by human chorionic gonadotrophin, when at least three follicles measured 18mm or more in diameter and serum estradiol concentration were at least 1000 ng/l [11].Oocyte retrieval was carried out by vaginal ultrasound guided follicular puncture, 36 h after HCG administration. Oocyte preparation for ICSI includes removal of the cumulus and corona radiata cells by hyaluronidase digestion. The oocyte was assessed under an inverted microscope, a healthy oocyte has intact ooplasm membrane, intact zona pellucida and clear cytoplasm. Being metaphase II oocyte, it has one polar body in the perivitelline space [12]. A single, living, immobilized spermatozoon was aspirated first into the injection pipette. The oocyte was fixed on the holding pipette in a way that the polar body is situated at 6 o'clock while the injection pipette is pushed through the zona pellucida at the 3 o'clock position and into the cytoplasm, where the sperm was delivered together with the smallest amount of medium. After the injection, the oocyte was washed and stored in 25 μl micro drops of culture medium in a petri dish and stored at 37°C in an incubator containing 5% CO2, 5% O2 and 90% N2 [13].Cases that had positive fertilization and embryo development had embryo transfer on day 3. The primary outcome was clinical pregnancy rate; defined as sonographic detection of intrauterine viable pregnancy. Secondary outcomes included fertilization rate, embryo development and embryo grade. Fertilization was defined as observation of two distinct pronuclei and two polar bodies 16-18 hours after micro-injection. Fertilization rate was calculated as the number of fertilized oocytes divided by the total number of retrieved mature oocytes for each couple. Fertilized oocytes were observed for embryo development on days 2 and 3 after micro-injection. Embryo development rate was calculated as the number of embryos developed divided by the total number of mature oocytes for each couple.Statistical AnalysisStatistical analysis was performed using SPSS for Windows version 17. Difference between two independent groups was analyzed using independent student’s t-test and mean difference (MD) with its 95% confidence interval (95% CI) [for numeric variables] or chi-squared test and odds ratio (OR) with its 95% CI [for categorical variables]. Significance level was set at 0.05.
3. Results
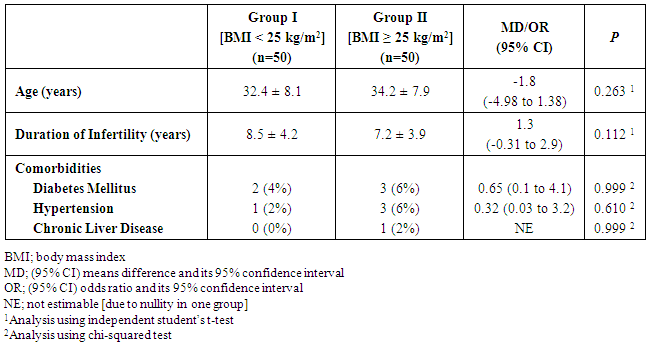
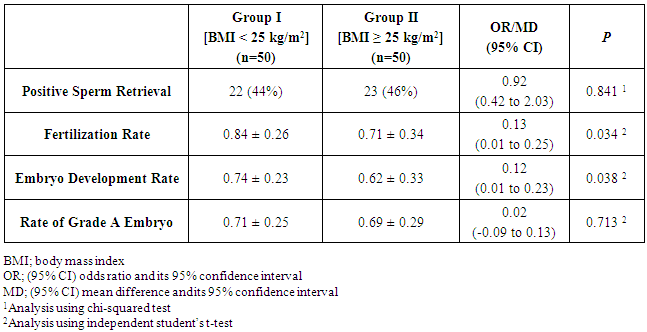
- Fifty patients planned to undergo TESE and ICSI for NOA were included in each group. There was no significant difference between patients of both groups regarding the age, duration of infertility and comorbidities (Table 1).
|
|
|
4. Discussion
- Despite the well-observed and known inverse relationship between female obesity and fertility, the relationship between male obesity and fertility has not been that extensively studied [14], particularly regarding the outcomes of sperm retrieval through TESE and assisted reproduction outcomes [15]. The current study showed that though male obesity was not associated with reduced sperm retrieval in male patients undergoing TESE for NOA. Several previous reports have shown similar observations. Ramasamy et al. reported, in a cohort study, comparable sperm retrieval rate in obese and non-obese men [15]. In a larger study over nearly 1,000 obese men with a BMI > 30 kg/m2, Sandlow found sperm retrieval rates similar to those reported in non-obese counterparts [16].The current study showed significant association between male obesity and reduced fertilization rate and embryo development rate. This finding was not in agreement to the results found by Merhi et al. [17] and Bakos et al. [18], they showed no significant impact of male obesity and early embryo development.Nevertheless, Petersen et al. [14] stated that male obesity seems to be associated with higher rates of sperm DNA damage, which may explain the reason of the significant adverse impact of male obesity on early embryo development observed in the current study. In addition, Ramlau-Hansen et al. [19] showed that male obesity was associated with reduced androgen and increased estrogen levels resulting in hormonal profile very similar to hypo-gonadotropic, hyper–estrogenic, hypo-androgenemia. The reduced clinical pregnancy rates in obese men included in the current study is probably explained in the same way though published data are rather conflicting. Ramasamy et al. [15] and Keltz et al. [20] showed that male obesity was associated with significant reduction in the chance of pregnancy. On the contrary, Braga et al. [21] and Thomsen et al. [22] showed no significant impact of male obesity on fertilization and pregnancy rates. The insignificant association between male obesity and embryo grade observed in the current study might be explained by the fact stated by Bakos et al. [23] that paternal genome is not activated until the four-to-eight cell stage in human.On the other hand, Thomsen et al. [22] stated that male overweight and obesity does not seem to have any negative impact on the outcome of IVF and ICSI in males patients in the reproductive age partnered with non-obese females.
5. Conclusions
- Among men with NOA, although TESE outcome is not affected, obesity seems to be associated with reduced TESE and ICSI outcomes in terms of reduced fertilization, embryo development and clinical pregnancy rates.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML