-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2017; 7(0): 356-362
doi:10.5923/j.ajmms.20170710.03

Assessment of Vitamin D Level in Sudanese Post Renal Transplant Patients – Khartoum State
Zainab S. A. Ali, Ikram H. M. Babiker, Ayman A. M. Alameen
Department of Chemical Pathology, Faculty of Medical Laboratory Sciences, University of Khartoum, Sudan
Correspondence to: Ayman A. M. Alameen, Department of Chemical Pathology, Faculty of Medical Laboratory Sciences, University of Khartoum, Sudan.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

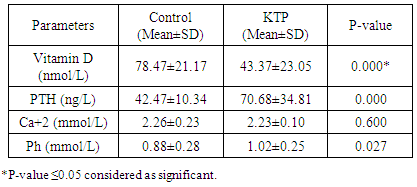
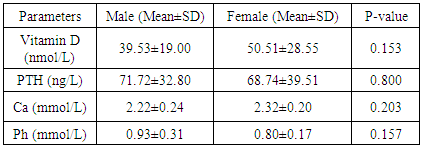
Background: Insufficient levels of Vitamin D and abnormal metabolism of calcium and phosphate are common problems among post renal transplant patients and general population. It creates a universal health apprehension since vitamin D deficiency is associated with many diseases. Vitamin D supplements after transplantation has been suggested to prevent risk of bone loss. Tacrolimus (FK506) and cyclosporine A (CsA), two kinds of immunosuppressive drugs, have been widely used in the field of renal transplantation. As triple-immunosuppressive regimen, FK506-Mycophenolate Mofetil (MMF)- Prednisone (Pred) or CsA-MMF-Pred was applied mainly depended on doctors’ experience and drug economics. However, there was no any detail treatment guidelines for the drug selection. The objectives of this study are to assess the prevalence of Vitamin D deficiency in the Sudanese renal transplant patients, compared with controls and study the role of immunosuppressive drugs. Methodology: This study was conducted in kidney transplantation centers to investigate the levels of Vitamin D, Parathyroid hormone, calcium and phosphorus and the effect of immunosuppressive drug on vitamin D metabolism in Sudanese renal-transplantation patients, Khartoum state during March 2017. The control group (n=40) matched for age and sex with renal post-transplants patients group (n=40). Results: In our study, 80 participants, 26 out of 40 individuals (65%) were males and 14 (35%) were females in each study group (patient and control). The mean age was 42.25±14.46 years in kidney transplant patients and 42.15±14.66 years in control group. Inadequate serum 25-hydroxyvitamin D (vitamin D level: 50 – 72.5 nmol/L) was seen in 17.5% of transplant patients and 35% of the controls, 72.5% of patients had vitamin D deficiency (level of vitamin D < 50 nmol/L) and 5% of control group. There was significant difference in vitamin D concentrations between the two groups (P value < 0.05). Low vitamin D concentrations were associated with increased Parathyroid Hormone (PTH) levels (P value < 0.05). Tacrolimus (FK-506) was associated with higher risk of deficiency. Conclusion: Vitamin D deficiency is prevalent among Sudanese kidney transplant patients; there were insufficient and deficient status among healthy control too, due to lack of exposure to sun despite being in a region with high UV light or poor diet. They may need vitamin D supplements for stabilization of serum vitamin D and improving the diet and life style.
Keywords: Vitamin D, PTH, Calcium, Phosphorus, Renal transplant
Cite this paper: Zainab S. A. Ali, Ikram H. M. Babiker, Ayman A. M. Alameen, Assessment of Vitamin D Level in Sudanese Post Renal Transplant Patients – Khartoum State, American Journal of Medicine and Medical Sciences, Vol. 7 No. 0, 2017, pp. 356-362. doi: 10.5923/j.ajmms.20170710.03.
Article Outline
1. Introduction
- High incidence of vitamin D insufficiency has been found in the general population, in patients with chronic kidney disease and kidney transplant patients. Kidney transplant patients have suppressed immune system which makes them more prone to chronic diseases than the other populations, due to the nonspecific immunosuppressive agents needed to prevent rejection of transplanted organ. Because of their immunosuppressive therapy, the patients are advised not to get direct exposure to the sun, to reduce the risk of skin carcinoma. The chronic diseases which are associated with kidney transplant include diabetes mellitus, cardiovascular diseases, cancers, and infectious diseases. Adequate vitamin D status has been linked to reducing the risk of many diseases [1-4].Vitamin D deficiency is common in children and adults and may increase the risk of bone fracture. Deficiency of Vitamin D has been involved with growth retardation and skeletal deformities in children and worsening the status of bone disorders such as osteopenia and osteoporosis and causes osteomalacia in adults. Vitamin D insufficiency mainly results from reduced skin synthesis due to lack of exposure to the sun, sunscreen use and poor Vitamin D diet. Sun exposure is required to satisfy human needs of the vitamin. Vitamin D is gained from an endogenous syntheses by UV light which are absorbed by 7-dehydrocholesterol in the skin, to produce pre-vitamin D3, which is converted to vitamin D3. Metabolism of Vitamin D3 occurs in the liver to form 25-hydroxyvitamin D3, and then in the kidney it is converted by the enzyme 1α-hydroxylase to the biologically active form 1, 25-dihydroxyvitamin D3 [5-7].Vitamin D functions in the intestine, bone, and kidney, plays a role in the metabolism of Calcium and Phosphate and regulates Parathyroid hormone levels. Vitamin D hormone production is strongly feedback regulated directly or indirectly by calcium and phosphorus levels of the plasma. The vitamin D is not solely responsible for the calcium and phosphorus. Phosphate concentrations are regulated by the action of Vitamin D, hormonal control by PTH and fibroblast growth factor 23 (FGF23) [8, 9]. PTH stimulates vitamin D synthesis in the kidney while vitamin D applies negative feedback on PTH secretion [10].Vitamin D deficiency is associated with the production of parathyroid hormone. PTH levels increase post-transplantation [11, 12]. Vitamin D deficiency is difficult in kidney transplant patients and related to use of steroids immunosuppressive drugs [13].This study was undertaken to assess the level of vitamin D among Sudanese renal post-transplant patients and assess the influence of immunosuppressive medications and other factors on vitamin D level.
2. Materials and Methods
- A cross-sectional case-control study was performed during period of March-May 2017. Eighty participants (40 patients and 40 healthy individuals) were involved with age ranged between (12-70 years). Approval was obtained from Clinical Pathology department, Research Board Review. Informed consent (written/verbal) was obtained from the participants after explaining the study and assuring the safety for participants and voluntary participation. Kidney transplant patients attended the outpatient nephrology centers, in Khartoum-Sudan were asked to participate. Sample and clinical information were used anonymously. Patients suffering from parathyroid gland disease, history of malignancies, autoimmune disease, administration of anticonvulsants and heparin, acute illness, chronic liver diseases, mental disorders, kidney dysfunction and need for dialysis were excluded. Also for control group individuals with kidney dysfunction, chronic liver disease, hypertension, mental illness and diabetes mellitus were excluded. All patients had stable kidney function. Data about using vitamin supplements, diet, life-style and sunscreen use were collected in a questionnaire. Vitamin D levels were classified as: sufficient: more than 30 ng/mL (>75 nmol/L), insufficient: 20 – 29 ng/mL (50-72.5 nmol/L), deficiency < 20 ng/mL (<50 nmol/L). Overnight fasting blood specimen was drawn, centrifuged at 3000 rpm for 10 min and stored at −20°C until utilized. Laboratory variables were measured including Vitamin D which estimated using Euroimmun 25-OH vitamin D competitive ELISA kits. PTH levels and tacrolimus were estimated using cobas e411, Germany. Cyclosporine was assessed using cobas integra 400. Calcium and phosphate were measured using Mindray BS-380.
2.1. Measurement of Vitamin D
- Solid phase competitive inhibition enzyme immunoassay was used to determine vitD (in use the vitD Elisa kit (E170314BD) EuroIMMUN AG) Germen. According to the manufacture protocols 200 μL of sample diluted by biotin added into Micro plate well which was coated with monoclonal anti vitD antibodies, during incubation antigen antibodies reaction occurred, then unbounded 25-OH vitD was removed by washing. 100μL of streptavidin-peroxidase was added to detect bound biotin labeled 25-OH vitD. 100μL of tetramethylbenzidine was added to promote a color reaction. The color intensity is inversely proportional 25-OH vitD concentration in the sample.
2.2. Statistical Analysis
- Statistical evaluation was performed using the Microsoft Office Excel (Microsoft Office Excel for windows; 2010) and SPSS (SPSS for windows version 22). Data from all patients were expressed as percentage and mean±SD, t-test was used to compare the mean concentration level of vitamin D and some bone markers among KTP (kidney transplant patients) and control, significance with p-value threshold <0.05. Pearson’s chi-square test was used to determine if there was a significant relationship between nominal variables. Significant correlation (r) was calculated using linear correlation test to study relationship between vitamin D and in-study variables.
3. Results
- The study contained equal number of patients (n=40) and control (n=40) with matched gender and age group. In our study, 26 (65%) participants were males and 14 (35%) were females in each study group (patient and control group) (Figure 1). The mean of age was 42.25 ± 14.47 for patients and 42.15 ± 14.66 for control group. Age grouped as <40 years old (37%) and >40 years old (63%) (Figure 2).
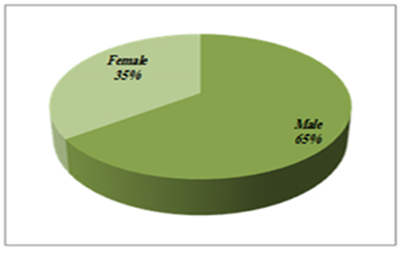 | Figure 1. Gender distribution among the study groups, matched for Kidney transplant patients and control |
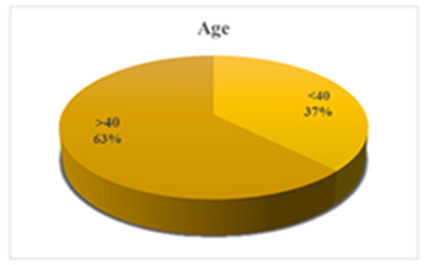 | Figure 2. Age divided as age <40 and >40 years old |
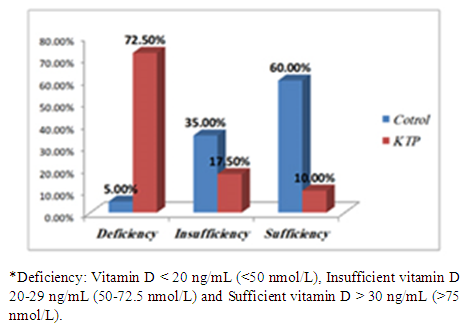 | Figure 3. Percentage and classification of Vitamin D status* |
|
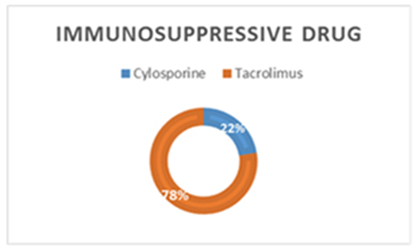 | Figure 4. Percentage of immunosuppressive drug users among patients |
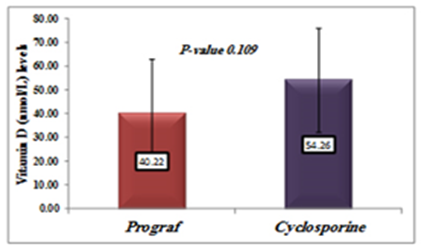 | Figure 5. Comparison between Vitamin D levels in patients treated with Tacrolimus (Prograf) and cyclosporine |
|
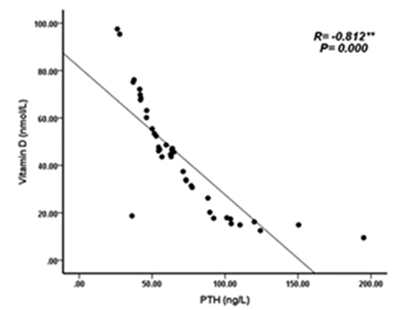 | Figure 6. Correlation between Vitamin D (nmol/L) levels and PTH (ng/L) in KTP |
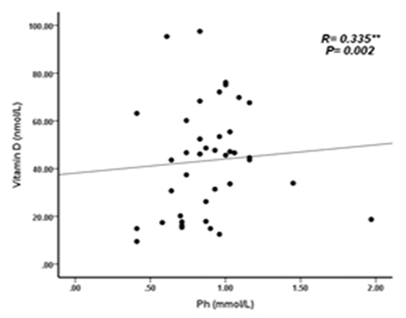 | Figure 7. Correlation between vitamin D (nmol/L) levels and Phosphate (mmol/L) among KTP |
|
4. Discussion
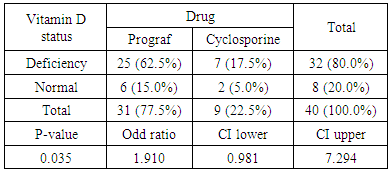
- Deficiency of Vitamin D has been involved with growth retardation and skeletal deformities in children and worsening the status of bone disorders such as osteopenia and osteoporosis and causes osteomalacia in adults [5-7]. There are different chronic diseases which associated with kidney transplant include diabetes mellitus, cardiovascular diseases, cancers, and infectious diseases. Adequate vitamin D status has been linked to reducing the risk of many diseases [1-4].In the present study, only 10% of patients in the case study had adequate blood levels of vitamin D and 72.5% had deficient levels of vitamin D this in concordance with a study done by Manju Aggarwal, et al reported that out of 51 patients; four patients (8%) were vitamin D sufficient, 17 patients (33%) insufficient, 26 patients (51%) mildly deficient, and four (8%) severely deficient [14]. Also recent study by Keyzer CA et al showed that 49% of long-term KT recipients were vitamin D deficient, 33% were insufficient and 18% were sufficient [15]. Other studies in western countries found similar results with (10%-20%) of patients had sufficient 25-OH-D level [16, 17]. Studies in Asian countries mentioned that inadequate vitamin D levels were prevalent in the study group [18, 19]. Study in Sudan by Osama Eltayeb M. Elkhider, et al showed that 21% of Sudanese population has vitamin D deficiency out of 74 apparently healthy subjects, 17 subjects (21.6%) had low vitamin D level and 11 subjects out of 17 with vitamin D deficiency [20]. The difference in levels from our study is probably due to insufficient diet, which was considered in the questionnaire, most of participants (patients and control) confessed that they do not eat many sources of vitamin D or calcium on daily basis and do not get enough exposure to the sun. The traditional clothing may play a factor; it covers skin from getting enough UV absorbance. Also as Sudan is an African country, various studies showed that darker skin colored individuals has lower vitamin D than whites, and vitamin D differs from a race to another [21-23].The findings in current study showed that Phosphate was significantly lower in patients compared to control group. Patients with stable graft function can still have hypophosphatemia, because relatively high FGF-23 concentration can lead to renal phosphate wasting and low serum phosphate in some patients [24] which is similar to a study performed by Bhan. I, et al that reported hypophosphatemia in 85% of subjects (<2.5 mg/dl), even after successful transplantation [25], also study by Sirilak S et al revealed low serum phosphate (2.5-4.5 mg/mL, 0.81-1.45 mmol/L) continued to present in long-term kidney transplantation and persistent high PTH had an influence on the mechanism [18]. In current study, PTH levels were elevated and inversely correlated with vitamin D this in concordance with Messa F et al study in which patients were put into groups, renal transplant group had PTH levels>80 pg/ml [26]. Another study by Sirilak S et al, reported persistent hyperparathyroidism [18]. Study done by Nasri H, et al found the mean iPTH was 18.4±8.2 Pg/mL their results revealed suppressed PTH secretion which disagreed with our results, this may be due to excessive intake of calcium and Vitamin D analogues, which may suppress PTH secretion [27].Vitamin D deficiency was associated with immunosuppressive treatment. Our results show no direct association between drug level and Vitamin D level among KTP in our study, yet higher Tacrolimus levels were associated with higher risk of vitamin D deficiency. These results were similar to a study conducted by Eyal O et al, they found that low vitamin D levels were associated with higher prescribed doses of Tacrolimus, yet there was no association between serum vitamin D and tacrolimus level [19]. Filipov JJ et al, found that serum 25(OH)D was inversely associated with calcineurin inhibitors (CNI) (p < 0.05) and unaffected by other immunosuppressive agents [28]. Lee CT et al reported elevated serum 1,25(OH)2 vitamin D without affecting the parathyroid hormone level rats injected with CsA (cyclosporine) and Tacrolimus [29]. The difference in findings in our study could be also due our small sample size and the limited prescription of Cyclosporine medication for patients. The drug is not prescribed for many patients anymore in Sudan, because it is nephrotoxic which remains a major limiting factor [30]. Many studies reported changes of vitamin D through seasons of the year [31, 32], and drugs metabolism show significant changes too. Lindh JD and coworkers reported that Tacrolimus levels showed seasonal variability that correlates with UV light-dependent changes in vitamin D [32]. The findings in our study show that vitamin D was positively correlated with phosphate level (P. value=0.002, r=0.335). Both patients and controls had calcium levels within acceptable range (8.5-10.5 mg/dl, 2.1-2.6 mmol/L). Secondary hyperparathyroidism had led to normalizing calcium levels by mobilizing calcium from bones and lowering phosphate level because of increased urinary excretion, and may lead to rickets and osteomalacia in adults in the long run [5].
5. Conclusions
- In this study, there was a high prevalence of vitamin D deficiency among renal transplant recipients. Therefore, the study laid weight on routine estimation of vitamin D and PTH. The use of active vitamin D with or without bisphosphonate is effective in preventing early post-transplant bone loss.
ACKNOWLEDGEMENTS
- Special thanks to our parents for being there for us all the way. We (authors) are grateful for the help from Ismail Mohamed Ismail Maryoud in the statistical analysis. We thank everyone who helped us in the study and all participated authors.
Declarations
- The research was approved as a project, a part of Master’s degree in Chemical Pathology – course program. The research was approved by Chemical Pathology Research Review Board – Faculty of Medical Laboratory Sciences – University of Khartoum. Acceptance from the nephrology clinic was obtained. Consent form was obtained from patients and control group after understanding the study.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML