-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2017; 7(0): 345-349
doi:10.5923/j.ajmms.20170710.01

Effect of Aqueous Root Extract of Citropsis articulata on the Levels of cAMP and cGMP – Dependent Protein Kinase-I in Penile Tissue of New Zealand White Male Rabbits
Eze Ejike Daniel1, Nganda Ponsiano1, Okpanachi Omachonu Alfred1, Sheu Oluwadare Sulaiman2, Ayikobua Emmanuel Tiyo1
1Department of Physiology, Faculty of Biomedical Sciences, Kampala International University, Kampala, Uganda
2Department of Physiology, Faculty of Medicine, Kampala International University, Dar es Salaam, Tanzania
Correspondence to: Eze Ejike Daniel, Department of Physiology, Faculty of Biomedical Sciences, Kampala International University, Kampala, Uganda.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background: The aqueous root extract of Citropsis articulata (C. articulata) has been shown to increase serum testosterone levels and intromission frequency. Aim: The aim of the study was to determine the effect of aqueous root extract of C. articulata on the levels of cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) – dependent protein kinase -I in penile tissues of New Zealand white male rabbits. Materials and Methods: The roots of C. articulata were picked from the wild and the aqueous extract was obtained. Adult New Zealand male white rabbits were immobilized by cervical dislocation and their spinal cord incised and the penile corpora cavernosa removed. Corpora cavernosa tissues were incubated with the extract or control drugs for 40 minutes at 37°C with continuous bubbling of oxygen (95%) and carbon dioxide (5%). Tissue levels of cAMP and cGMP – dependent protein kinase-I in the penile tissues were determined using ELISA method. Results: The aqueous root extract of C. articulata caused a significant (P< 0.05) increase in penile tissue levels of cGMP and cAMP dependent protein kinase-I when compared with the baseline control. Conclusion: The elevated levels of cAMP and cGMP–dependent protein kinase-I in penile tissues by the extract may be adduced to the presence of secondary metabolites in C. articulata root, which may act either singly or synergistically.
Keywords: Citropsis articulata, cGMP–dependent protein kinase-I, cAMP–dependent protein kinase-I, Erectile dysfunction, Penile tissue
Cite this paper: Eze Ejike Daniel, Nganda Ponsiano, Okpanachi Omachonu Alfred, Sheu Oluwadare Sulaiman, Ayikobua Emmanuel Tiyo, Effect of Aqueous Root Extract of Citropsis articulata on the Levels of cAMP and cGMP – Dependent Protein Kinase-I in Penile Tissue of New Zealand White Male Rabbits, American Journal of Medicine and Medical Sciences, Vol. 7 No. 0, 2017, pp. 345-349. doi: 10.5923/j.ajmms.20170710.01.
1. Introduction
- Erectile dysfunction (ED) is the continuous inability to attain or maintain an erection firm enough for satisfactory sexual intercourse. It is common in men between the ages of 40 and 70 years [1]. ED can be caused by hormonal deficiencies [2]; psychological factors such as depression and physical factors such as heart disease, diabetes and high blood pressure [3]. ED is a common and widespread health problem that affects an estimated range of 15 million to 30 million men worldwide [4]. In Africa, the prevalence of men reporting ED related problems in primary health care clinics is estimated at 57.4% in Nigeria, 63.6% in Egypt and 80.8% in Ethiopia [2]. Many orthodox pharmaceutical therapies have been available for treatment of ED and include Tadalafil, Sildenafil and Vardenafil [2]; urethral suppositories, vacuum devices and penile implants [5]. However, these methods are expensive and not readily available thus, medicinal plants have been used as an alternative [6]. In Uganda, the plants commonly used to treat ED include Mondia whitei, Citropsis articulata, Ekebergia capensis and Cola acuminate [6]. C. articulata from the Rutaceae family, widely known as African cherry orange is a small citrus fruit commonly found in south western Uganda [7], and is used primarily as a medicinal herb. The crude aqueous leaf extract of C. articulata was shown to increase serum testosterone and luteinizing hormone (LH) [7]. Its root extract increased mounting and intromission frequency [8]. C. articulata contains saponins, proteins, free amino acids, arginine and steroid glycosides [9]. Arginine is the substrate for NOS which synthesizes NO and activates the cGMP pathway [10]. Penile erection is regulated by two cellular pathways, the cGMP/NO pathway and the cAMP pathway [11]. The penile cavernous tissues contain nitric oxide synthase (NOS) which synthesize NO from L-arginine [12]. The NO released diffuses into the smooth muscle cells where it directly activates soluble guanylate cyclase leading to a rise in intracellular concentrations of cGMP [12]. The cGMP formed activates cGMP dependent protein kinase type 1 (PKG-I), which also activates various target proteins in the corpus cavernosa smooth muscle [13]. This leads to a decrease in intracellular Ca2+ and reduced sensitivity of the contractile proteins (myosin and actin) to Ca2+ [14]. The corpora cavernosa are also richly supplied with autonomic nerves which when stimulated release vaso-active intestinal peptide (VIP) [15]. VIP stimulates VIP receptors which are coupled to a G - protein adenylyl cyclase which catalyses the conversation of AMP to cAMP [16]. The c-AMP then activates protein kinase-A (cAK). The activated c-AK phosphorylates phospholamban, a protein which normally inhibits the Ca2+ pump within the membrane of the sarcoplasmic reticulum [17]. The Ca2+ pump is then activated and the level of cytoplasmic Ca2+ is reduced resulting in smooth muscle relaxation [18]. Therefore, the present study assessed the penile tissue levels of cAMP and cGMP-dependent protein kinase-I of New Zealand white male rabbits after in vitro administration of aqueous root extract of C. articulata.
2. Materials and Methods
- Drugs Nitric oxide (sodium nitropruside) prostaglandin E1 (PGE1 alcohol), cAMP and PGK-I (cGKIα) were procured from Santa – Cruz Biotechnology, Germany. C. articulata aqueous root extract was dissolved in distilled water.Plant Collection, Identification and ExtractionRoots of C. articulata plant were collected from the wild at Omukiyenje village, Masha subcounty, Isingiro district, South Western Uganda. A herbarium specimen was prepared and taken for identification in the Herbarium Unit, College of Natural Sciences Makerere University, Uganda, where a voucher specimen number (PTRL016) was given. The aqueous root extract of C. articulata was obtained using a method described by Gakunga [7]. The dried roots were crushed into a fine powder using a mortar and pestle. About 1.5 kg of the fine powder from the roots was boiled to extract the active components (hot maceration). The mixture was cooled and filtered using cotton wool and was further finely filtered using Whatman No: 1 filter paper. The filtrate was concentrated with a rotary evaporator at a temperature of 50°C and later at room temperature, the resultant concentrate was dried in an oven at a temperature of 40°C until a crystalline solid was obtained and stored before use.Experimental AnimalsTen (10) adult male rabbits, 12 months old (New Zealand white) weighing between 3 – 4kg were procured from the Animal House of School of Veterinary Medicine and Animal Resources, College of Veterinary Medicine, Animal Resources and Bio-security, Makerere University, Uganda. They were housed in cages (2 animals per cage) under a 12 – hour light: dark cycle. The animals were acclimatized for seven days during which they were fed on standard pellet diet and were provided with water ad libitum.Dissection of Corpora Cavernosa TissuesThe rabbits were immobilized by cervical dislocation and their spinal cord incised. The entire penis was surgically removed and rapidly placed in Dulbecco’s modified Eagle’s medium (DMEM) with oxygen 95% and carbondioxide 5% bubbled through following a method already described by Xiao et al [19]. The corpora cavernosa was carefully dissected from the penis by removing surrounding adipose tissue, connective tissue and the surrounding tunica albuginea under a light microscope [19]. The penile tissues were kept in DMEM medium with oxygen 95% and carbondioxide 5% bubbled through. Each penis was dissected longitudinally into two strips and the two strips were cut into two segments measuring about 2mm by 2mm by 10mm.Experimental ProcedureThe penile tissues harvested were clustered into four groups with each group having six tissues (n = 6). These were incubated in 6 – well plates with 0.5mL of DMEM at 37°C for one hour. After DMEM solution was removed, the segments in each group were treated as follows.Group I (Baseline group), to the six portions of penile tissues in 6 – well plates 0.2mL of DMEM was added and incubated at 37°C for 40 minutes.Group II (Experimental group), to the six portions of penile tissues in 6 – well plates 0.2mL of DMEM + 0.2ml of 2.0 mg/ml aqueous root extract of C. articulata (final concentration) was added and incubated at 37°C for 40 minutes.Group III (cAMP group, Positive control), to the six portions of corpus cavernosum in 12 – well plates 0.2mL of DMEM + 0.1ml of Prostaglandin E1 (20µM) was added and incubated at 37°C for 40 minutes. Prostaglandin E1 activate guanyl cyclase which stimulates cAMP production in Corpus cavernosum tissues [11].Group IV (PKG -I group, Positive control) to the six portions of penile tissues in 12 – well plates 0.1mL of DMEM + 0.1ml of 1µmol/L of Sodium Nitropruside [20] was added and incubated at 37°C for 40 minutes. Sodium nitropruside is a donor of NO thus it activates the NO/cGMP pathway [16]. After incubation, the different portions of penile tissues were rapidly frozen in liquid nitrogen and stored at -80°C. The frozen tissues were weighed and 200µL of 0.1M hydrochloric acid was added. The tissues were then homogenized on ice and centrifuged at top speed for 5 minutes at room temperature. The supernatant was collected and stored at -80°C. 100µL of the supernatant per sample was used to determine levels of PGKI and cAMP using direct ELISA (Santa – Cruz Laboratories, Germany). The supernatant was incubated at 4°C for eight hours in multi well plates to bind the antigen to the wells. The excess antigen was washed away and primary polyclonal antibodies to PGKI and cAMP were covalently attached to PGKI and cAMP respectively by incubation at room temperature for three hour. The excess antibody was washed away and secondary antibodies were covalently attached to the primary antibody by incubation at room temperature for one hour. The excess reagents were washed away and a substrate was added. After a short incubation time the reaction was stopped and the yellow colour generated was read using a spectrophotometer multi-plate reader at 450nm. The intensity of the bound colour (optical density) was directly proportional to the concentration of PGKI and cAMP in the sample.Data AnalysisThe optical density of cAMP and PGK-I from the six groups was expressed as Mean ± SD and was subjected to one way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test to determine significant differences between groups. The obtained data were presented in form of bar charts. The statistical significance level was defined as P< 0.05.Ethical ConsiderationsThe guidelines to ethical research provided by the Uganda National Council for Science and Technology (UNCST) were adhered to during the research period. Approval was sought from the Department of Physiology, and the Directorate of Postgraduate Studies and Research, Kampala International University (KIU), Uganda, before commencement of the study. Minimizing the number of rabbits to be used in the study was emphasized as well as proper disposal of research materials.
3. Results
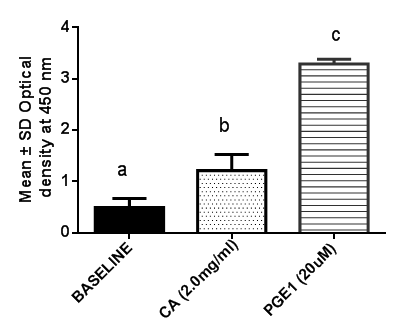
- Regarding cAMP, aqueous root extract of C. articulata at a dose concentration of 2.0mg/ml significantly (P <0.05) increased the cAMP levels (optical density) in penile tissues when compared with the base line control group. However, PGE1 (20µM) administration significantly (P<0.05) resulted to a higher cAMP levels when compared with the baseline control group, indicating that the reference drug has better activity than the extract at the dose administered (Figure 1).
4. Discussion
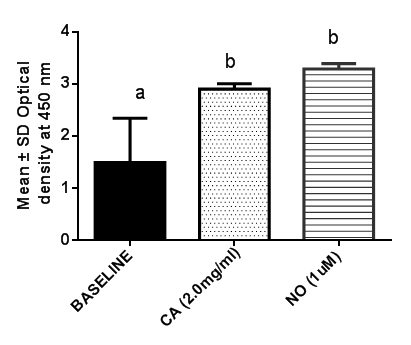
- The effect of C. articulata on levels of testosterone and mounting and intromission frequency has been previously reported [21, 9]. However, the mechanism through which penile erection occur has not been validated. This study presents the in vitro effect of C. articulata on levels of cAMP and cGMP – dependent protein kinase-I in penile tissues of New Zealand white male rabbits. C. articulata contains saponins, proteins, free amino acids, arginine and steroid glycosides [9]. The action of these biomolecules on smooth muscles may involve the modulation of different cellular pathways [22]. The results showed that the aqueous root extract of C. articulata caused a significant (P < 0.05) increase in levels of PGK-I in penile tissues when compared to the baseline control. This increase is an indication of enhanced production of cGMP which activates PGK-I. The increased levels of PGK-I are attributed to the effect of the amino acid arginine one of the phytochemicals in C.articulata, which is converted to nitric oxide by NOS in penile tissues. Nitric oxide directly activates soluble guanylate cyclase leading to a rise in intracellular concentrations of cGMP and PGKI in smooth muscle cells [23]. The results also showed that the aqueous root extract of C. articulata caused an increase in levels of cAMP in penile tissues. Smooth muscle cells have prostaglandin E1 (PGE1), prostaglandin E2 (PGE2), β – adrenergic, vasoactive intestinal petide (VIP) and corticotropin releasing hormone (CRH) receptors [24]. Binding of any agonist on one of these receptors activates all isoforms of adenylate cyclase to produce cAMP from ATP [25]. The increase in PGK-I and cAMP due to C. articulata bserved in this study could facilitate relaxation relaxation of the blood vessels and smooth muscles of the penis. Thus promoting erection.
5. Conclusion
- The increased cAMP and PGK-I levels in penile tissues by the extract may be attributed to the presence of phytochemicals in the aqueous root extract of C. articulata, which may act either singly or synergistically.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML