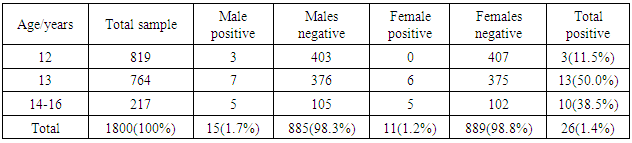-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2017; 7(9): 338-343
doi:10.5923/j.ajmms.20170709.03

Tribal Distribution of Aggressive Periodontitis in Sudanese School Children (12-16 years) in Khartoum State
Abdalla Saleh Aljabry, Abdalraouf Ali Aloutabi
Department of Periodontology, Faculty of Dentistry, University of Medical Sciences and Technology, Khartoum, Sudan
Correspondence to: Abdalla Saleh Aljabry, Department of Periodontology, Faculty of Dentistry, University of Medical Sciences and Technology, Khartoum, Sudan.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Introduction: aggressive periodontitis is apparently prevalent in Sudan but epidemiological baseline data concerning the condition is not adequate. Aim: this research was conducted to estimate the prevalence and gender distribution in a group of Sudanese children. Materials and methods: equal number of males and females were selected randomly from the school children list of the Ministry of Education of Khartoum State. The age range of the selected subjects was 12-16 years. A total number of 1800 subjects were included. Clinical examination was done using ordinary chair, sunlight and Williams’s periodontal probe. First molars and central incisors were probed; those with attachment loss ≥ 4mm were considered positive for aggressive periodontitis and were radiographed using periapical films for confirmation. Results: 26 children (15 males/11 females) were found positive for aggressive periodontitis. Prevalence is 1.7% and 1.2% for males and females, respectively. The prevalence was 1.4%. The disease was discovered in seven tribes. Those of pure African origins showed higher prevalence rates than other ethenities. Discussion: The overall prevalence and the gender distribution are within the range found in previous studies. The study sample showed wide variety of ethnic groups and this may explain why the disease was more prevalent among some tribes. Conclusion: aggressive periodontitis is prevalent in Sudan. The disease is more prevalent in the more Negroid tribes. More researches may be needed among other social groups to further clarify this tribal distribution of the disease. Considering control of the disease, tribes with higher prevalence should be specially targeted. General dentists should improve their clinical skills to diagnose this disease in its early stages. The school health programs should include screening for aggressive periodontitis as a routine health examination.
Keywords: Aggressive periodontitis, School children, Khartoum state
Cite this paper: Abdalla Saleh Aljabry, Abdalraouf Ali Aloutabi, Tribal Distribution of Aggressive Periodontitis in Sudanese School Children (12-16 years) in Khartoum State, American Journal of Medicine and Medical Sciences, Vol. 7 No. 9, 2017, pp. 338-343. doi: 10.5923/j.ajmms.20170709.03.
1. Introduction
- Aggressive periodontitis is a relatively new term adopted by the American Academy of Periodontology to describe conditions previously described as early-onset periodontitis, juvenile periodontitis, prepubertal periodontitis and rapidly progressive periodontitis. [1] It is a disease with multifactorial etiology that affects the periodontal attachment apparatus. It has a strong familial and racial influence; [2] It runs in families and is more common in blacks. It is usually associated with certain subgingival microflora and altered immune response. [3] In addition, interactions between the bacteria and the host factors, together with the environmental (e.g. cigarette smoking) and genetically controlled (e.g. IgG2 response to Aggregatobacter actinomycetemcomitans) modifying factors may contribute to determining the specific clinical manifestation of disease. [4] Aggressive periodontitis is either localized (less than 30% of sites affected) or generalized (more than 30% of sites affected). [5] Great variations exist between the two categories. The localized form usually involves the first permanent molars and /or incisors while the generalized pattern involves these teeth plus other teeth in the oral cavity. Bone loss is usually angular and the mesial surface is more frequently affected. Early diagnosis of the disease is a key factor in the establishment of a better prognosis.There is only few published information on the prevalence of these conditions in any Sudanese population, therefore more studies are needed. Therefore, the objective of this study was to determine the prevalence of aggressive periodontitis among Sudanese school children in Khartoum state.
2. Materials and Methods
- A sample of 1800 school children (900 male and 900 female) were included in the study (Figure 1).
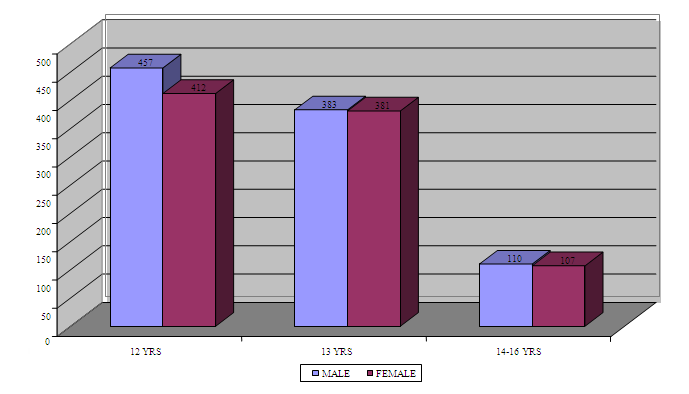 | Figure 1. Distribution of male and female children among different age groups (N = 1800) |
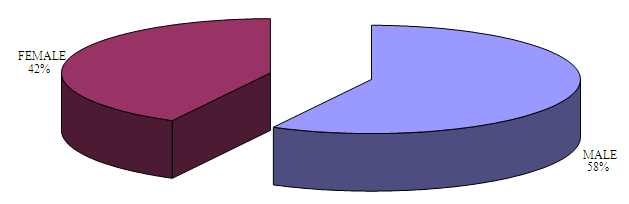 | Figure 2. Distribution of the positive cases (26) among male (15) and female (11) children |
|
3. Results
- A total sample size of 1800 child were examined (Figure 1). A total number of 26 cases were diagnosed positive for the disease. The distribution of positive cases within gender is presented in Figure 2. The prevalence of aggressive periodontitis is 1.7% for males and 1.2% for females. The overall prevalence in this study group is 1.4% (Table 2).
|
|
|
|
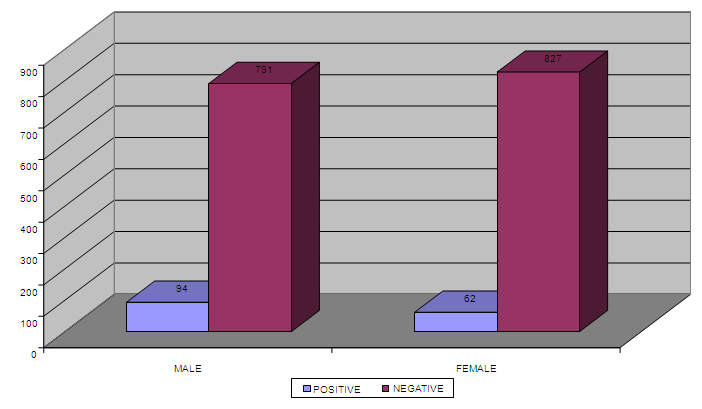 | Figure 3. Presence of calculus among the males and females in the Negative cases (No, =1774) |
4. Discussion
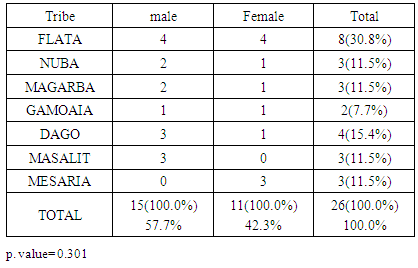
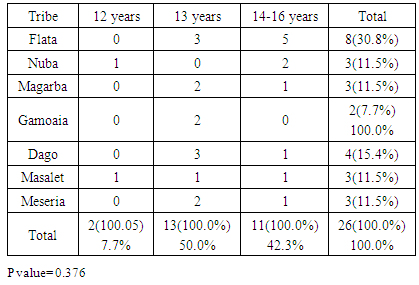
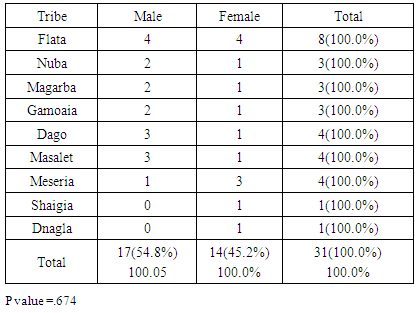
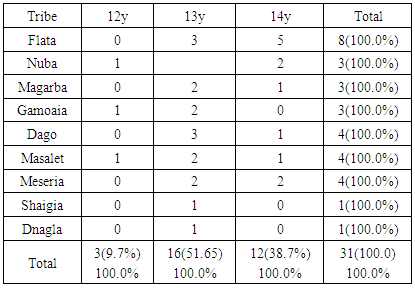
- The clinical diagnosis of the positive cases was confirmed with periapical X-ray films. Bone destruction was localized to the first molars and incisor teeth which is a diagnostic criterion for aggressive periodontitis. The results of this study estimate the prevalence of aggressive periodontitis in Sudan as 1.4%. This rate falls within the international figures which estimate the prevalence of this disease in the western countries as less than 1%, while it is as high as 6.5% in Africa (Uganda). [7] It resembles the prevalence in Nigeria (1.56%) but lesser than that among African-Americans (2.1%) [8]. Gender wise, males in our study group are slightly more affected: Female to male ratio is 0.9:1. This holds true for black Americans. [9] Prevalence is higher in the Sudanese tribes of pure African origins compared to other ethenities. The diversity of ethnic groups in Sudan is well presented in this study population.The distribution of positive cases in relation to tribes and age groups shows that both males and females can be equally affected (Tables 3 and 4). Previous studies have shown variations in prevalence in the females in different societies. [10] These variations could be explained by variations in the race and age of the populations screened, differences in diagnostic criteria used to determine the presence and extent of the disease together with the demographic and socioeconomic variables [11]. In one study done in Saudi subjects in Makah, the prevalence was found to be 0.42%, where female to male ratio was found to be 1.88:1 [12]. In a study in a Finish population, female to male ratio was reported to be 1.7:1. [13] While in a study in a mixed population, Albandar reported 3.6:1 female to male ratio. [14] Melvin et al reported overall prevalence rate of juvenile periodontitis in young racially mixed population to be 1.1:1 female to male ratio. [15] It is apparent that variations exist in the prevalence of the disease in these studies and females are consistently more affected than males.In our study, the disease was generally more prevalent in males of the same tribe. However, it was noticed that in Meseria tribe (a less Negroid tribe), no disease was detected among males while 3 females had the disease with a prevalence of 27.3% of the total diseased females (11.5% of the total prevalence). Therefore, a reversed pattern of disease predisposition exists in Meseria tribe. In the Dajo tribe, 3 males showed the disease while females were free of it. This accounts for 20% of the diseased males and 11.5% of the total prevalence. Some tribes showed 2:1 male to female ratio while in the Flata tribe the ratio was 1:1. The highest prevalence was found among Flata tribe; 8 cases (4 males and 4 females), which accounts for 30.8% of the total prevalence. Internationally, the difference in male to female ratio is generally mild. It has been suggested that both sexes are equally affected if confounding factors are considered. [16]Periodontitis implies destruction in the periodontal attachment apparatus. Clinical attachment loss is measured in mm. Studies on the prevalence of aggressive periodontitis used 3mm to 5mm to establish the diagnosis of periodontitis. [17, 18]In this study, attachment loss of 4 mm is used to establish the diagnosis. To investigate the effect of using various level of attachment loss as basis for diagnosis, we have also analyzed the study sample using 3 mm as a basis for diagnosis. As expected, the prevalence rate increased to 1.7% and 2 new tribes were added (table 4 & 5). All positive cases showed moderate plaque accumulation. Plaque is also present in the oral surfaces of the negative cases. Plaque accumulation was minimal in the age group 12 and 13 years, while moderate in the age group 12-14 years. Therefore, results of this study support the theory that plaque is not the only cause factor for the development of the disease, and that other factors contribute. Interestingly, it was noted that even at this young age, females are more alerted about their oral hygiene than their male counterparts, a matter which was demonstrated by less plaque on the females' teeth.Inflammation is not always a feature of aggressive periodontitis. [19] In the present study population, gingivae around the central incisors and first molars of most of the positive cases showed little signs of inflammation. Only one patient was presented with severe inflammation. For the negative cases, when gingival index in relation to age and gender is compared with that for positive cases, most of the children in age group 12 and 13 years presented with healthy gingivae while 206 children in the age group 14-16 showed mild inflammation. Some cases in the age group 14-16 showed moderate inflammation which may coincide with their age, where hormonal changes may render their gingivae more prone to inflammation. [20] Some of the positive cases showed calculus in relation to the central incisors and first molars, while the majority of the negative cases also had calculus deposition. This may indicate that the role of calculus in the pathogenesis of aggressive periodontitis is minor. Females presented with deeper pockets in the central incisors, while males had deeper pockets related to their first molars. This may be due to the fact that central incisors appear earlier in females while the first molars erupt earlier in males. [21] Attachment loss is more severe in males than females. This holds true for the incisors and first molars. High readings (6 and 7 mms) were recorded by females around their incisors while males showed more Attachment loss in relation to their first molars. As for the pocket depth and recession, attachment loss is also severe in the older age group (14-16 years) because more destruction occurs with time. Fewer females showed recession in relation to the incisors compared to males (eight males, two females). For the first molars, only one female showed recession while eight males presented with recession around these teeth. This may be due to the fact that females are usually more careful and more promoted about their appearance which is reflected in the better oral hygiene of the females compared to males.The age group 14-16 years showed more recession than the younger age groups both for the incisors and first molars. Recession is almost absent in the age group 12 years while the age group 13 showed some recession. With the advancement of the disease process, more recession occurs.More studies are required in other geographical areas to find out the prevalence in the country as a whole. It is important that school health programs should include screening for aggressive periodontitis as a routine health examination. Special programs should target these tribes with higher prevalence of the disease, to ensure early diagnoses and better management. Dentists should improve their diagnostic skills to diagnose cases with aggressive periodontitis in its early stage.
5. Conclusions
- The prevalence of localized aggressive periodontitis in this selected population of school children is similar to prevalence rates in populations in different developing countries but it is higher than the prevalence rates in the western populations. Most of the positive cases were not aware about their problem. Tribes of African ethenities showed higher disease prevalence.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML