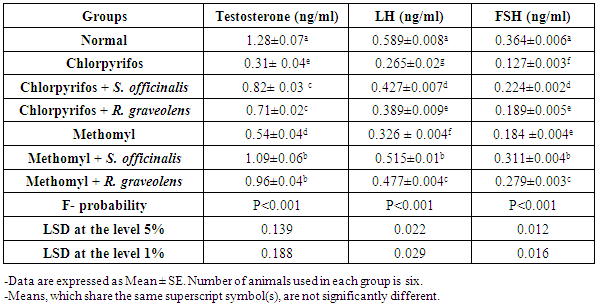
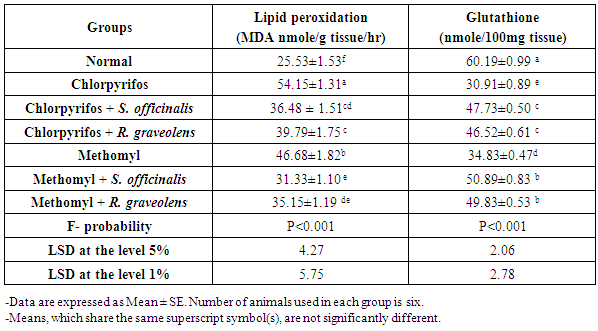
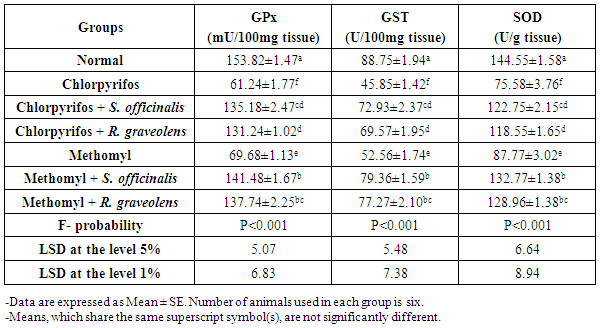
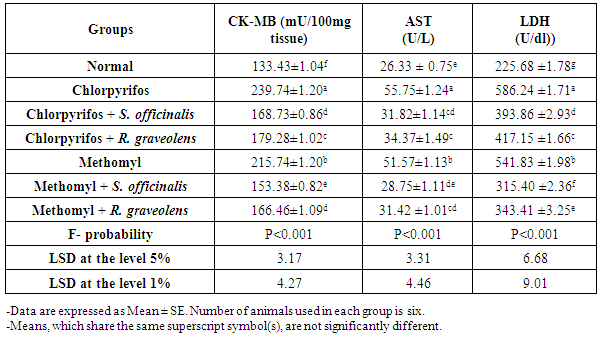
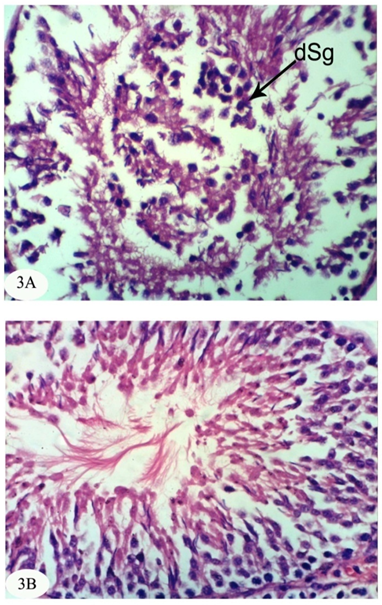
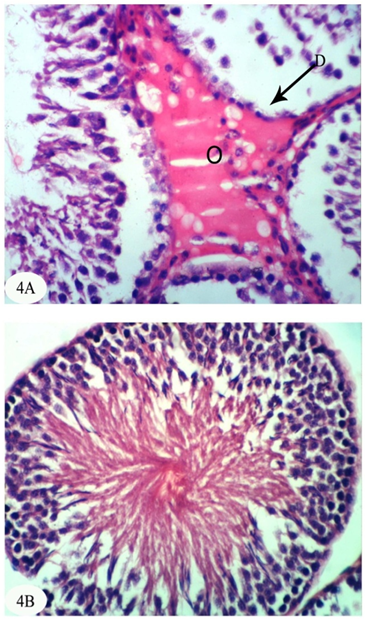
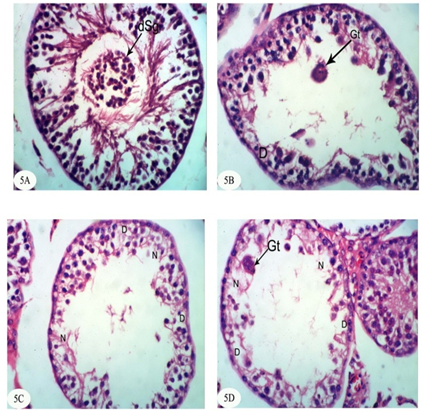
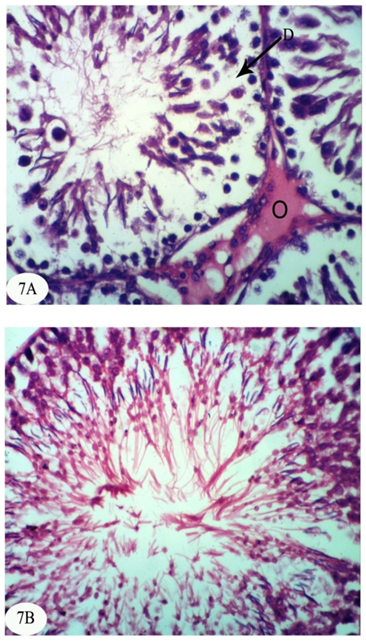
| [1] | Dawson AH, Eddleston M, Senarathna L, Mohamed F, Gawarammana I, Bowe SJ, Manuweera G, Buckley NA. Acute human lethal toxicity of agricultural pesticides: a prospective cohort study. PLoS Medicine, 2010, 7:e1000357. |
| [2] | Lee SJ, Mehler L, Beckman J, Diebolt-Brown B, Prado J, Lackovic M, Waltz J, Mulay P, Schwartz A, Mitchell Y, Moraga-McHaley S, Gergely R, Calvert GM. Acute pesticide illnesses associated with off-target pesticide drift from agricultural applications: 11 States, 1998-2006. Environmental Health Perspectives, 2011, 119:1162-1169. |
| [3] | Pan-Germany. Pesticide and health hazards. Facts and figures. 2012, 1-16. |
| [4] | Abdollahi M, Ranjbar A, Shadnia S, Nikfar S, Rezaie A. Pesticides and oxidative stress: a review. Med Sci Monit., 2004, 10 (6): 141-147. |
| [5] | Agrawal A, Sharma B. Pesticides induced oxidative stress in mammalian systems: Review Article. Int. J Biol Med Res., 2010, 1(3): 90-104. |
| [6] | Salih, N. F. Jaafar, M. S. Heavy metals in blood and urine impact on the woman fertility. Chem. Mat. Res., 2013, 3(3): 81-89. |
| [7] | Meeker, J. D.; Ryan, L.; Barr, D. B. and Hauser, R. Exposure to nonpersistent insecticides and male reproductive hormones. Epidemiol., 2006, 17(1): 61–68. |
| [8] | Sangha GK, Kaur K, Kher, KS. Cypremethrine induced pathological and biochemical changes in reproductive organs of female rats. J. Environ. Biol., 2013, 34(1): 99-105. |
| [9] | Wesseling C, Aragon A, Castillo L, Corriols M, Chaverri F, de la Cruz E, Keifer M, Monge P, Partanen TJ, Ruepert C, van Wendel de Joode B. Hazardous pesticides in Central America. Int. J. Occup. Environ. Health., 2001, 7(4): 287-294. |
| [10] | Konradsen F, Van der Hoek W, Cole DC, Hutchinson G, Daisley H, Singh S, Eddleston M. Reducing acute poisoning in developing countries-options for restricting the availability of pesticides. Toxicol., 2003, 192(2-3): 249-261. |
| [11] | Coronado GD, Thompson B, Strong L, Griffith WC, Islas I. Agricultural task and exposure to organophosphate pesticides among farm workers. Environ. Health. Persp., 2004, 112(2): 142-147. |
| [12] | Soares W, Almeida RM, Moro S. Rural work and risk factors associated with pesticide use in Minas Gerais. Brazil. Cad. Saude. Publica., 2003, 19(4): 1117-1127. |
| [13] | Mancini F, Van Bruggen AHC, Jiggins JLS, Ambatipudi AC, Murphy H. Acute pesticide poisoning among female and male cotton growers in India. Int. J. Occup. Environ. Health., 2005, 11(3): 221-232. |
| [14] | Remor AP, Totti CC, Moreira DA, Dutra GP, Heuser VD, Boeira JM. Occupational exposure of farm workers to pesticides: biochemical parameters and evaluation of genotoxicity. Environ. Int., 2009, 35(2): 273-278. |
| [15] | Hurtig AK, San Sebastian M, Soto A, Shingre A, Zambrano D, Guerrero W. Pesticide use among farmers in the Amazon basin of Ecuador. Arch. Environ. Health., 2003, 58(4): 223-228. |
| [16] | Atreya K. Health costs from short-term exposure to pesticides in Nepal. Soc. Sci. Med., 2008, 67(4): 511-519. |
| [17] | Cuvelier ME, Berset C, Richard, H. Antioxidant constituents in sage (Salvia officinalis). Khimiya Prirodnykh Soedineii., 1994, 5: 686–7. |
| [18] | Baricevic D, Bartol T. The biological/pharmacological activity of the Salvia genus. In: Kintzios SE, editor. Sage: the genus salvia. Amsterdam: Harwood Academic Publishers, Netherlands, 2000, 143– 84. |
| [19] | Zupko I, Hohmann J, Redei D, Falkay G, Janicsak G, Mathe, I. Antioxidant activity of leaves of Salvia species in enzyme-dependent and enzyme-independent systems of lipid peroxidation and their phenolic constituents. Planta Med., 2001, 97: 383–389. |
| [20] | Capasso R, Izzo AI, Capasso F, Romussi G, Bisto A, Mascolo N. A diterpenoid from Salvia cinnabarina inhibits mouse intestinal motility in vivo. Planta. Med., 2004, 70(4): 375–377. |
| [21] | Kamatou GP, Makunga NP, Ramogola WPN, Viljoen AM. South African salvia species: a review of biological activities and phytochemistry. J. Ethnopharm., 2008, 119(3): 664-672. |
| [22] | Mitic-culafi CD, Vukovic-Gacic B, Knezevic-Vukcevic J, Stankovic S, Simic D. Comparative study on the antibacterial activity of volatiles from sage (Salvia officinalis L.). Arch. Biol. Sci., 2005, 57(3): 173-178. |
| [23] | Longaray Delamare AP, Moschen-Pistorello IT, Artico L, Atti-Serafini L, Echeverrigaray S. Antibacterial activity of the essential oils of Salvia officinalis L. and Salvia triloba L. cultivated in South Brazil. Food. Chem., 2007, 100: 603-608. |
| [24] | Smidling D, Mitic-Culafic D, Vukovic-Gacic B, Simic D, Knezevic-Vukcevic J. Evaluation of antiviral activity of fractionated extracts of sage Salvia officinalis L. (Lamiaceae). Arch. Biol. Sci., 2008, 60(1): 421-429. |
| [25] | Baricevic D, Sosa S, Della Loggia R, Tubaro A, Simonovska B, Krasna A, Zupancic A. Topical anti-inflamatory activity of Salvia officinalis L. leaves: the relevance of ursalic acid. J. Ethnopharmacol., 2001, 75(2-3): 125-132. |
| [26] | Eidi M, Eidi A, Zamanizadeh H. Effect of Salvia officinalis L. leaves on serum glucose and insulin in healthy and streptozotocin-induced diabetic rats. J. Ethnopharmacol., 2005, 100: 310-313. |
| [27] | Hasanein P, Felehgari Z, Emamjomeh A. Preventive effects of Salvia officinalis L. against learning and memory deficit induced by diabetes in rats: Possible hypoglycaemic and antioxidant mechanisms. Neurosci Lett., 2016, 622:72-7. |
| [28] | Bouaziz M, Yangui T, Sayadi S, Dhouib A. properties of essential oils from Salvia officinalis L. cultivated in Tunisia. Food Chem. Toxicol., 2009, 47(11): 2755-2760. |
| [29] | Vukovic-Gacic B, Nikcevic S, Beric-Bjedov T, Knezevic-Vukcevic J, Simic D. Antimutagenic effect of essential oil of sage (Salvia officinalis L.) and its monoterpenes against UV-induced mutations in Escherichia coli and Saccharomyces cerevisiae. Food Chem. Toxicol., 2006, 44(10): 1730-1738. |
| [30] | Xavier CP, Lima CF, Fernandes-Ferreira M, Pereira-Wilson C. Salvia fruticosa, Salvia officinalis and rosmarinic acid induce apoptosis and inhibit proliferation of human colorectal cell lines: the role in MAPK/ERK pathway. Nutr. Cancer, 2009, 61(4): 564-571. |
| [31] | Parsai1 A, Eidi M, Sadeghipour A. Hepatoprotective effect of sage (Salvia officinalis L.) leaves hydro-methanolic extract against Aspergillus Parasticus aflatoxin-induced liver damage in male rats. Bulletin of Pharmaceutical Research, 2014, 4(3):129-32. |
| [32] | Ratheesh M, Helen A. Anti-inflammatory activity of Ruta graveolens Linn on carrageenan induced paw edema in wistar male rats. Afr. J. Biotechnol., 2007, 6(10): 1209-1211. |
| [33] | Kuzovkina I, Al'terma, I, Schneider B. Specific accumulation and revised structures of acridone alkaloid glucosides in the tips of transformed roots of Ruta graveolens. Phytochemistry, 2004, 65(8): 1095-1100. |
| [34] | Loonat F, Amabeoku GJ. Antinociceptive, anti-inflammatory and antipyretic activities of the leaf methanol extract of Ruta graveolens L. (Rutaceae) in mice and rats. Afr J Tradit Complement Altern Med., 2014, 11(3):173-81. |
| [35] | Tarique M, Siddiqui HH, Khushtar M, Rahman MA. Protective effect of hydro-alcoholic extract of Ruta graveolens Linn. leaves on indomethacin and pylorus ligation-induced gastric ulcer in rats. J Ayurveda Integr Med., 2016, 7(1):38-43. |
| [36] | Toserkani A, Jalali MR, Najafzaheh H. Changes of lipid profiles, glucose, and hemogram after administration of Ruta graveolens extract in diabetic rats. Comp. Clin. Pathol., 2011, 10: 1331-1333. |
| [37] | Meepagala KM, Schrader KK, Wedge DE, Duke SO. Algicidal and antifungal compounds from the roots of Ruta graveolens and synthesis of their analogs. Phytochemistry, 2005, 66(22): 2689-2695. |
| [38] | Khouri NA, EL-Akawi Z. Antiandrogenic activity of Ruta graveolens L in male albino rats with emphasis on sexual and aggressive behavior. Neuro Endocrinol. Lett., 2005, 26(6): 823-829. |
| [39] | FAO/WHO. Evaluation Report on Chlorpyrifos. 2006, Annex 1: Hazard Summary Provided By The Proposer. |
| [40] | FAO. FAO Specifications and Evaluations for Plant Protection Products. Food and Agriculture Organization/The United Nations. Methomyl Evaluation. 2002, Pp. 18. |
| [41] | Eidi M, Eidi A, Bahar M. Effects of Salvia officinalis L. (sage) leaves on memory retention and its interaction with the cholinergic system in rats. Nutrition, 2006, 22: 321–326. |
| [42] | Pandey P, Mehta A, Hajra S. Anti-diarrhoeal activity of ethanolic extracts of Ruta graveolens leaves and stem. Asian. J Pharm Clin Res., 2012, 5(4): 65-68. |
| [43] | Kachmar JF, Moss DW. In Fundamentals of Clinical Chemistry, 2nd ed. NW Tietz, Editor. WB Saunders, Philadephia, 1976, Pp. 682. |
| [44] | Schumann G, Klauke R. New IFCC reference procedures for the determination of catalytic activity concentrations of five enzymes in serum: preliminary upper reference limits obtained in hospitalized subjects. Clin. Chem. Acta., 2003, 327: 69-79. |
| [45] | Henderson AR, Moss DW. Enzymes: In Teitz Fundamentals of Clinical Chemistry, 5 Ed. Burtis, C.A. & Ashwood, E.R. editors (W. B. Saunders eds. Philadelphia USA). 2001, pp. 352. |
| [46] | Huang HF, Marshall GR, Rosenberg R, Nieschlag E. Restoration of spermatogenesis by high levels of testosterone in hypophysectomised rats after long-term regression. Acta Endocrinol., 1987, 116(4): 433–444. |
| [47] | Beutler E, Duron O, Kelly BM. Improved method for determination of blood glutathione. J Lab Clin Med., 1963, 61: 882-888. |
| [48] | Preuss HG, Jarrel ST, Scheckenbach R, Lieberman S, Anderson RA. Comparative effects of chromium, vanadium and gymnema sylvestre on sugar-induced blood pressure elevations in SHR. J Am Coll Nutr., 1998, 17(2): 116-123. |
| [49] | Matkovics B, Sasvari M, Kotorman M, Varga IS, Hai, DQ, Varga, C. Further prove on oxidative stress in alloxan diabetic rat tissues. Acta Physiol Hung., 1997/1998, 85(3): 183-192. |
| [50] | Mannervik B, Gutenberg C. Glutathione transeferase (Human Placenta). Methods Enzymol., 1981, 77: 231-235. |
| [51] | Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and convenient assay for superoxide dismutase. Eur J Biochem., 1974, 47: 469-474. |
| [52] | PC-STAT. one-way analysis of variance. Version 1 A (C) Copyright. The University of Georgia. Programs coded by Roa, M.; Blane K. and Zonneberg, M., University of Georgia, USA, 1985. |
| [53] | Kang HG, Jeong SH, Cho JH, Kim DG, Park JM, Cho MH. Chlorpyrifos-methyl shows anti-androgenic activity without estrogenic activity in rats. Toxicology, 2004, 199(2-3): 219-230. |
| [54] | Jeong S, Kim B, Kang H, Ku H, Cho J. Effect of chlorpyrifos-methyl on steroid and thyroid hormones in rat F0-and F1-generations. Toxicology, 2006, 220(2-3): 189-202. |
| [55] | Joshi SC, Mathur R, Gulati N. Testicular toxicity of chlorpirifos (an organophosphate pesticide) in albino rat. Toxicology Industrial Health., 2007, 23(7): 439-444. |
| [56] | Olorunshola KV, Achie LN, Akpomiemie ML. Ascorbic acid ameliorates toxic effects of chlopyrifos on testicular functions of albino rats. British Journal of Pharmacology and Toxicology, 2011, 2(5): 262-269. |
| [57] | Attia AA, ElMazoudy RA, El-Shenawy NS. Antioxidant role of propolis extract against oxidative damage of testicular tissue induced by insecticide chlorpyrifos in rats. Pesticide Biochemistry and Physiology, 2012, 103: 87–93. |
| [58] | Ikpeme EV, Okonko LE, Udensi OU. Detrimental effects of chlorpyrifos and cypermethrin on reproductive physiology of male albino rats. Research Journal of Environmental Toxicology, 2016, 10 (1): 68-74. |
| [59] | Mahgoub AA, Mednay AH. Evaluation of chronic exposure of male rat reproductive system to insecticide methomyl. Pharmacological Research., 2001, 44(2): 73-80. |
| [60] | Shalaby M A, El Zorba H Y, Ziada RM. Reproductive toxicity of methomyl insecticide in male rats and protective effect of folic acid. Food and Chemical Toxicology, 2010, 48(11): 3221-3226. |
| [61] | Jalili S, Farshid AA, Heydari R, Ilkhanipour M, Salehi S. Histopathological observations on protective effects of vitamin E on endosulfan induced cardiotoxicity in rats. Pak J Biol Sci., 2007, 1:10(11): 1922-1925. |
| [62] | Al-Attar AM. The ameliorative role of β-carotene pretreatment on diazinon-induced enzymological and histopathological changes in wistar male rats. Global Journal of Pharmacology, 2009, 3(3): 171-177. |
| [63] | Al-Attar AM, Al-Taisan WA. Preventive effects of black seed (Nigella sativa) extract on Sprague Dawley rats exposed to diazinon. Australian Journal of Basic and Applied Sciences, 2010, 4(5): 957-968. |
| [64] | BAS H, Kalender Y. Chlorpyrifos induced cardiotoxicity in rats and the protective role of quercetin and catechin. Gazi University Journal of Science, 2011, 24(3):387-395. |
| [65] | Al-Sowayan NS, Mahmoud NH. The protective effect of grape seed extract on cardiotoxicity induced by doxorubicin drug in male rats. Advances in Bioscience and Biotechnology, 2014, 5, 1078-1089. |
| [66] | Razavi BM, Hosseinzadeh H, Imenshahidi M, Malekian M, Ramezani M, Abnous K. Evaluation of protein ubiquitylation in heart tissue of rats exposed to diazinon (an organophosphate insecticide) and crocin (an active saffron ingredient): role of HIF-1α. Drug Res (Stuttg)., 2015, 65(11): 561-6. |
| [67] | Farag RS, Badei AZMA, Hewedi FM, El-Barot GSA. Antioxidant activity of some spice essential oils on linoleic acid oxidation in aqueous media. J Am Oil Chem Soc., 1989, 66: 792–9. |
| [68] | Steinmetz KA, Potter JD. Vegetables, fruit, and cancer prevention: a review. J Am Diet Assoc., 1996, 96(10): 1027-39. |
| [69] | Nees AR, Powles JW. Fruit and vegetables and cardiovascular disease: a review. Int J Epidemiol., 1997, 26(1): 1-13. |
| [70] | Aruoma OI. Free radicals, oxidative stress, and antioxidants in human health and disease. J Am Oil Chem.Soc., 1998, 75(2): 199-212. |
| [71] | Keli SO, Hertog MG, Feskens EJ, Kromhout D. Dietary flavonoids, antioxidant vitamins, and incidence of stroke: the Zutphen study. Arch Intern Med., 1996, 156: 637-642. |
| [72] | Pace-Asciak CR, Hahn S, Diamandis EP. The red wine phenolics trans-resveratrol and quercetin block human platelet aggregation and eicosanoid synthesis: implications for protection against coronary heart disease. Clin Chem Acta., 1995, 235: 207-219. |
| [73] | Chopra K, Singh M. Involvement of oxygen free radicals in cardioprotective effect of rutin A natyrally occurred flavonoid. Indian J Pharmacol., 1994, 26: 13-18. |
| [74] | Bergamini CM, Gambetti S, Dondi A, Cervellati C. Oxygen, reactive oxygen species and tissue damage. Curr Pharm Des., 2004, 10(14): 1611-1626. |
| [75] | Gultekin F, Delibas N, Yasar S, Kilinc I. In vivo changes in antioxidant systems and protective role of melatonin and a combination of vitamin C and vitamin E on oxidative damage in erythrocytes induced by chlorpyrifos-ethyl in rats. Arch Toxicol., 2001, 75(2): 88-96. |
| [76] | Gupta RC. Toxicology of organophosphates and carbamate compounds, (Gupta, RC. ed) Academic Press Elsevier, Amesrerdam Pp. 2006, 1-733. |
| [77] | Ran Q, Liang H, Ikeno Y, Qi W, Prolla TA, Roberts LJ, Wolf N, Van Remmen H, Richardson A. Reduction in glutathione peroxidase four increases life span through increased sensitivity to apoptosis. J Gerontol Series A: Biol Sci Med Sci., 2007, 62(9): 932-942. |
| [78] | Jett DA, Navo RV. In vitro and in vivo effects of chlorpyrifos on glutathione peroxidase and catalase in developing rat brain, Neurotoxicology, 2000, 21(1-2): 141-145. |
| [79] | Gultekin F, Ozturk M, Akdogan M. The effect of organophosphate insecticide chlorpyrifos-ethyl on lipid peroxidation and antioxidant enzymes (in vitro). Arch ToxicoI., 2000, 74(9): 533-538. |
| [80] | El-Shenawy NS, Al-Eisa RA. Mechanism of organophosphorus insecticide chlorpyrifos toxicity in isolated rat hepatocytes. J Egypt Soc Toxicol., 2010, 43: 87-112. |
| [81] | Goel A, Dani V, Dhawan DK. Protective effects of zinc on lipid peroxidation, antioxidant enzymes and hepatic histoarchitecture in chlorpyrifos-induced toxicity. Chem Biol Interact., 2005, 156(2-3): 131-140. |
| [82] | Aly N, EL-Gendy K, Mahmoud F, El-Sebae AK. Protective effect of vitamin C against chlorpyrifos oxidative stress in male mice, Pest Biochem & Physiol., 2010, 97(1): 7-12. |
| [83] | Altuntas I, Delibas N, Dou, DK, Ozmen S, Gultekin F. Role of reactive oxygen species in organophosphate insecticide phosalone toxicity in erythtocytes in vitro. Toxicol In Vitro., 2003, 17(2): 153-157. |
| [84] | Verma RS, Srivastava N. Effect of chlorpyrifos on thiobarbituric acid reactive substances, scavenging enzymes and gluthatione in rat tissues. Indian J Biochem Biophys., 2003, 40(6): 423-428. |
| [85] | Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annual Rev Pharmacol Toxicol., 2005, 45: 51-88. |
| [86] | Lee-Hilz YY, Boerboom AM, Westphal AH, Berkel WJ, Aarts JM, Rietjens IM. Pro-oxidant activity of flavonoids induces EpRE-mediated gene expression. Che Res Toxicol., 2006, 19(11): 1499-1505. |
| [87] | Saulsbury MD, Heyliger SO, Wang K, Johnson DJ. Chlorpyrifos induces oxidative stress in oligodendrocyte progenitor cells. Toxicology, 2009, 259(1-2): 1-9. |
| [88] | Mansour SA, Mossa AH. Lipid peroxidation and oxidative stress in rat erythrocytes induced by chlorpyrifos and the protective effect of zinc. Pesticide Biochemistry and Physiology, 2009, 93 (1): 34-39. |
| [89] | Sharma M, Pillai KK, Husain SZ, Giri DK. Protective role of propolis against alcohol carbon-tetrachloride induced hepatotoxicity in rat. Ind. J. Pharm., 1997, 29: 76–78. |
| [90] | Santos-Gomes PC, Seabra RM, Andrade PB, Fernandes-Ferreira M. Phenolic antioxidant compounds produced by in vitro shoots of sage (Salvia officinalis L.). Plant Sci., 2002, 162(6): 981-987. |
| [91] | Grzegorczyk I, Matkowski A, Wysokinska H. Antioxidant activity of extracts from in vitro cultures of Salvia officinalis L. Food Chem., 2007, 104: 536-541. |
| [92] | Patenkovic A, Stamenkovic-Radak M, Banjanac T, Andjelkovic M. Antimutagenic effect of sage tea in the wing spot test of Drosophila melanogaster. Food Chem. Toxicol., 2009, 47(1): 180-183. |
| [93] | Alkan F, Gursel F, Ates A, Zyurek M, Guclu K, Altun M. Protective effects of Salvia officinalis extract against cyclophosphamide-induced genotoxicity and oxidative stress in rats. Turk. J. Vet. Anim. Sci., 2012, 36(6): 646-654. |
| [94] | Affanas,s EVIB, Dorozkvo AI, Brodskii AV, Kostyuk VA, Potapovitch AI. Chelating and free radical scavenging mechanisms of inhibitory action of rutin and quercetin in lipid peroxidation. Biochem. Pharmcol., 1989, 38: 1763-1769. |
| [95] | Hanasaki Y, Ogawa S, Fukui, S. The correlation between active oxygens scavenging and antioxidative of flavonoids free radical. Biol. Med., 1994, 16: 845-850. |
| [96] | Horvathova K, Novotny L, Vachalkova A. The free radical scavenging activity of four flavonoids determined by the comet assay. Neoplasma, 2003, 50(4): 291-295. |
| [97] | Park YC, Rimbach G, Saliou C, Valacchi G, Packer L. Activity of monomeric, diameric and trimeric flavonoids on NO production, TNF-α secretion and NF-kappaB-depentent gene expression in RAW 264.7 macrophages. FEBS. Lett., 2000, 464(2-3): 93-97. |
| [98] | Birt DF, Hendrich S, Wang W. Dietary agents in cancer prevention: flavonoids and isoflavonoids. Pharmacol. Ther., 2001, 90: 157-177. |
| [99] | Van der logt EM, Roelofs HM, Nagengast FM, Peters WH. Induction of rat hepatic and intestinal UDP glucuronosyl-transferases by naturally occurring dietary anticarcinogens. Carcinogensis, 2003, 24: 1651-1656. |
| [100] | Park SY, Bok SH, Jeon SM, Park YB, Lee SJ, Jeong TS, Choi MS. Effect of rutin and tannic acid supplements on cholesterol metabolism in rats. Nutr. Res., 2002, 22: 283-295. |
| [101] | Sheu JR, Hsiao G, Chou PH, Shen MY, Chou DS. Mechanisms involved in the antiplatelet activity of rutin, a glycoside of the flavonol quercetin, in human platelets. J. Agric. Food Chem., 2004, 52: 4414-4418. |


 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML