-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2017; 7(6): 265-270
doi:10.5923/j.ajmms.20170706.05

Studies on Malaria Parasite and Haemoglobin Level among Pregnant Women Attending Antenatal at Benue State General Hospital, Otukpo, Nigeria
Peter Adikwu1, E. U. Amuta1, G. A. Obande2, A. O. Adulugba3, Emmanuel Abba3
1Department of Biological Sciences, Federal University of Agriculture, Makurdi, Nigeria
2Department of Microbiology, Federal University, Lafia, Nigeria
3Department of Science Laboratory Technology, Benue State Polytechnic, Ugbokolo, Nigeria
Correspondence to: Peter Adikwu, Department of Biological Sciences, Federal University of Agriculture, Makurdi, Nigeria.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

A study was conducted to investigate the relationship between malaria and anaemia in pregnant women attending antenatal care at General Hospital Otukpo. A total of three hundred and fifty (350) pregnant women who granted informed consent were recruited for the study. Demographic information such as their age, number of previous pregnancies and age of present pregnancy were collected using structured questionnaire. Blood samples collected by venipuncture were used to prepare Giemsa-stained smears for determination of the presence of malaria parasite in the pregnant women while haemoglobin level was read on a Hawksley microhaematocrit reader using blood samples collected into capillary tubes and centrifuged at 3000rpm. Collected data were analysed using SPSS version 20. Results showed that malaria was higher in the youngest (80.4%) and oldest (66.7%) age groups while anaemia was higher in the younger age groups of 15 – 20 (78.4%) and 21 – 26 (40.9%). Malaria, anaemia and combination of both were highest in primigravid (84.0%, 86.0% and 82.0%) and least in multigravid (34.0%, 10.5% and 9.0%) and age of pregnancy was found to influence the rate of malaria (X2 (2) 85.469 = .000), anaemia (X2 (2) 22.450 = .000). A statistically significant difference was also observed between the haemoglobin level of malaria infected and non-infected pregnant women (p<0.05) and age group 27-33 years was observed to have had the widest difference between the Hb level in malaria infected (29%) and the non-infected (37%) pregnant women. The study posits that a large population of pregnant women in the study area may be at risk of malaria and anaemia related problems associated with child bearing and underscores the need for integrated prevention and control measures to reduce mortality and morbidity in pregnant women.
Keywords: Malaria, Anaemia, Prevalence, Gravid, Trimester
Cite this paper: Peter Adikwu, E. U. Amuta, G. A. Obande, A. O. Adulugba, Emmanuel Abba, Studies on Malaria Parasite and Haemoglobin Level among Pregnant Women Attending Antenatal at Benue State General Hospital, Otukpo, Nigeria, American Journal of Medicine and Medical Sciences, Vol. 7 No. 6, 2017, pp. 265-270. doi: 10.5923/j.ajmms.20170706.05.
1. Introduction
- Malaria is a mosquito-borne infectious disease of humans and other animals caused by parasitic protozoans of the genus Plasmodium (Ukaegbu et al., 2014). This plasmodial infectious disease transmitted through the bite of an infected female anopheles mosquito (Monif et al., 2004), is one of the most devastating infectious diseases in tropical and subtropical developing countries of the world with more than one million deaths annually (Schantz-Dunn et al., 2009). Malaria debilitates and kills more people than any other single infectious disease (Sherman, 1998). It is considered a disease of the poor and duly recognized as a public health problem with overwhelming medical, social and economic implications (Isah et al., 2011). It has contributed to a huge economic loss of about 12 billion US dollars annually from medical cost, loss of man-hours, school absenteeism and other indirect costs in Nigeria (NMCP/RBM, 2009).Malaria in pregnancy is a major public health concern in Nigeria. It is also a major cause of maternal morbidity and mortality (Bergstorm et al., 2004; Erhabor et al., 2010). Pregnant women, children and immunocompromised individuals have the highest morbidity and mortality, and Africa bears the heaviest burden due to malaria (Schantz-Dunn et al., 2009). Malaria and pregnancy are mutually aggravating conditions; the physiological and pathological changes in pregnancy due to malaria have a synergistic effect on the course of each other. This is due to the fact that pregnancy is a period of drastic physiological change which places extreme stress on various systems of the body. Complications resulting from malaria in pregnancy are still birth, foetal complications resulting from high placental plasmodia burdens, new born death and low birth weight in infants (Mbah et al., 2015). Anaemia has been implicated as a leading factor resulting in the complications associated with malaria morbidity and mortality (Achidi et al., 2005). The most common consequence of Plasmodium falciparum malaria infection is maternal anaemia (Uneke, 2008; Adesina et al., 2009). It is estimated that about 200,000 to 500,000 pregnant women develop severe anaemia as a result of malaria in Sub-saharan Africa (Adesina et al., 2009, Steketee et al., 2001). Although, malaria is an important contributor in anaemia, it is pertinent to also know that anaemia is multifactorial in origin. Deficiencies in nutrition, HIV infection, hookworm and sickle cell (genetic red blood cell disorder) are other important contributing factors (Adesina et al., 2009, Shankar, 2000).Malaria in endemic areas such as Nigeria is difficult to define due to asymptomatic infection even among pregnant women. In medical practice, the diagnosis of malaria based on fever and parasitaemia alone can be misleading resulting in gross over-diagnosis or under-diagnosis as well as inappropriate prescription and use of anti-malarias (Dicko et al., 2005). The accumulation/build-up of plasmodial parasites in the placenta makes them unavailable in the peripheral blood; the patients will not demonstrate obvious symptoms and would therefore see no need for malarial diagnosis and treatment (Mbah et al., 2015).Given the known complications of malaria in pregnancy, even in the asymptomatic state, and the paucity of information on the above subject matter in the study area, this study was targeted at investigating antenatal malaria parasitaemia in relation to haemoglobin profile, gravidity and trimester of pregnant women in Otukpo Local Government Area, Benue State, Nigeria.
2. Methodology
- The study was conducted at the antenatal clinic of Benue State General Hospital, Otukpo between February and June, 2006. The hospital attends to inhabitants in the town, as well as those from surrounding towns and communities. It also serves as a referral centre for health facilities in the Benue South Senatorial district which is made up of nine (9) local government areas. The study area has two distinct seasons: a characteristic rainy season between April and October with high rainfalls in June, July and August, and dry season between December to February, mostly if not completely, devoid of rains. The study area is characterised by transmission of malaria all year round. Study populationPregnant women who came for antenatal were studied. Only those who provided informed consent after counselling were enrolled into the study. Three hundred and fifty pregnant women were randomly recruited. Structured questionnaire was used to obtain information such as Age, Trimester, Number of Pregnancy (Gravidae), Number of birth (parity) and Number of still births, Abortions or miscarriages.Sample collection and evaluationFive millilitres (5 ml) of blood was collected by venipuncture using aseptic techniques from each participant into two EDTA bottles. One bottle was used for diagnosis of malaria while the other was used to determine haemoglobin level.Diagnosis of malaria and determination of haemoglobin levelThick and thin films were prepared and stained with Giemsa stain for parasite identification and quantification as described by Jombo et al. (2011). Haemoglobin level was determined by centrifuging blood samples collected into capillary tubes at 3000rpm as described by Jombo et al. (2011). Readings were taken using the Hawksley microhaematocrit reader and recorded in percentage.Statistical analysisStatistical Package for Social Sciences (SPSS version 19) was used to generate descriptive data and analyse other parameters. Chi-square test was used to determine association between malaria and anaemia in pregnant women in relation to factors such as age, number of previous pregnancies and trimester of present pregnancy. Mean haemoglobin level of infected and non-infected pregnant women was compared using paired sample t-test. A p-value of <0.05 was considered statistically significant in all statistical comparisons.
3. Results
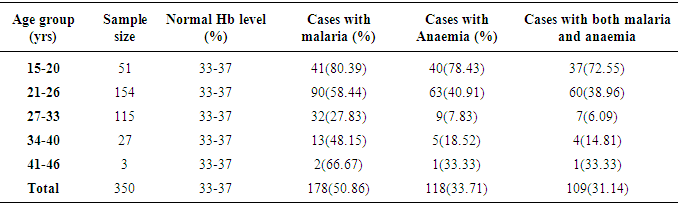
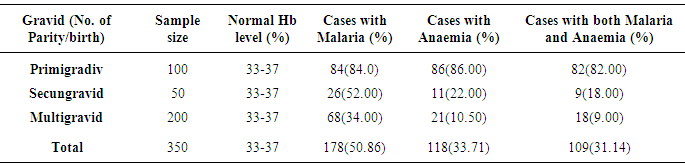
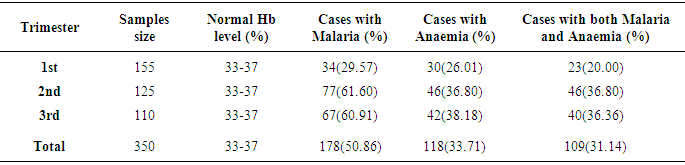
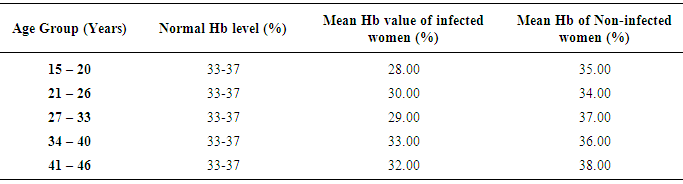
- Table 1 shows the prevalence of malaria parasite and anaemia in pregnant women in relation to age. Malaria was higher in the youngest (80.4%) and oldest (66.7%) age groups while anaemia was higher in the younger age groups of 15 – 20 (78.4%) and 21 – 26 (40.9%), than in the older age groups. Malaria and anaemia were least prevalent among age groups 27 – 33 (27.8% and 7.8%) and 34 – 40 (48.2% and 18.5%) respectively. There were statistically significant differences in cases of malaria (X2 (4) 46.131 = .000), anaemia (X2 (4) 86.479 = .000) and a mixture of malaria and anaemia (X2 (4) 82.195 = .000) in relation to age of pregnant women (α = .05).The prevalence of malaria and anaemia with relation to gravid is as presented in Table 2. Prevalence of malaria and anaemia as well as the combination of both reduced with an increase in the number of previous births by pregnant women. Malaria, anaemia and combination of both were highest in primigravid (84.0%, 86.0% and 82.0%) and least in multigravid (34.0%, 10.5% and 9.0%). A statistically significant difference was observed between gravidity and the rate of malaria (X2 (2) 173.629 = .000), anaemia (X2 (2) 66.717 = .000) and combination of both (X2 (2) 170.370 = .000).Prevalence of malaria and anaemia with relation to trimester was also determined (Table 3). Prevalence of malaria and anaemia as well as the combination of both were observed to increase with age of pregnancy. Malaria, anaemia and combination of both were least in the first trimester of pregnancy (29.6%, 26.0% and 20.0%) and highest in the third trimester of pregnancy (60.9%, 38.2% and 36.45%). Age of pregnancy was found to influence the rate of malaria (X2 (2) 85.469 = .000), anaemia (X2 (2) 22.450 = .000) and combination of both malaria and anaemia (X2 (2) 31.435 = .000).Table 4 compares the haemoglobin level in infected and non-infected pregnant women. Mean haemoglobin level was generally lower in pregnant women infected with malaria than in uninfected pregnant women. There was a statistically significant difference between the haemoglobin level of malaria infected and non-infected pregnant women (t (4)6.039 = .004, p<0.05, α = .05). Age group 27-33 years had the widest difference between the Hb level in malaria infected (29%) and the non-infected (37%) pregnant women. The Hb level was however, not a function of the patient’s age (X2 (4) 5.000 = .287).
|
|
|
|
4. Discussion
- The study shows that the prevalence of malaria in pregnancy was higher in the youngest (80.4%) and oldest (66.7%) age groups with a significant association with anaemia. This is in agreement with the findings of previous studies (Mockenhaupt et al., 2000; Wakibara et al., 1997; Van et al., 2000; Mariella et al., 2003 and Jenavine et al., 2015). This observation could be attributed to the fact that the youngest age groups are immunologically naive to malaria infection since they were having malaria infection in pregnancy for the first time. Age and weaker immune status may have also played a role in increased incidence of malaria in women within the oldest age group, thereby predisposing them to malaria infection. Lander et al. (2012) also recorded high prevalence of malaria infection and anaemia among younger study subjects. He opined that prevalence reduced as the women advanced in age due to acquired immunity. According to earlier studies, the cause of anaemia could be associated with many risk factors which may include hook worm infestation, lack of iron intake and malaria parasitaemia (Jenavine et al., 2015; Brookers et al., 2008; Aikawa, 1988). Plasmodium falciparum was identified as the major cause of malaria within the study population. Jombo et al. (2011) also reported in an earlier study that 98% of malaria parasitemia in in pregnant women in Otukpo was caused by P. falciparum.The findings of this research have also demonstrated that primigravid women are more susceptible to malaria infection (84%) and anaemia (86%). This corroborates the findings of Jenavine et al. (2015), Bankole et al. (2012) and Adefionye et al. (2007) in similar researches within Ebonyi, Benin City and Osogbo respectively. A possible explanation to this is that the immune system of the primigravid women may not have been previously exposed to the physiological and pathological changes in pregnancy associated with malaria. With successive pregnancies, women are exposed to variety of strains of malaria parasite, and may develop efficient mechanism to control infection and prevent the disease. Hence, women getting pregnant for the first time (primigravid) have lower immunity against strains of malaria parasite and present more frequently with malaria (Bankole et al. 2012). The opposite is the case with women who have been pregnant before. The risk of infection reduces with increasing exposure to pregnancy resulting from acquisition of specific immunity to placental malaria arising from previous exposure. Some other report associated decrease in malaria susceptibility in multiparous women to development of anti-adhesion antibodies. These anti-adhesion antibodies are believed to protect against maternal malaria by acting against chondronitin sulphate A-binding parasites. They however, develop only over successive pregnancies and as such are not present in primigravid women (Duffy and Fried, 1999). Knowledge may have also influenced the rate of malaria infection. Although there is no data in this study to support this assertion, Amuta et al. (2014) opined that mutigravid women are more informed about prevention and management methods for malaria than primigravid women, leading to reduced prevalence within the group. A study by Agomo and Oyibo (2013) however did not establish any relationship between level of education and malaria in pregnant women in Lagos, Nigeria.Similarly, studies conducted by Nwagha et al. (2009), Amadi et al. (2000) and Barbin et al. (1991) also demonstrated that malaria and anaemia are more prevalent in primigravid and least prevalent in multigravid. The difference in prevalence rate in anaemia and malaria between primigravid and multigravid women in this study was statistically significant (p<0.05). This may be due to the recovery or adjustment of the multigravid’s immune system as parity increased. This leads to the women regaining immunity against parasite invasion, leading to reduced malaria and anaemia. Factors such as decreased iron from tissue, malnutrition and insufficient consumption of iron have been identified as causes of anaemia in pregnant women (Veghari et al., 2007). It is also known that haemolysis of parasitized red blood cells occurs in malaria infection. This leads to a reduction in red blood cells especially if the body’s replacement of those being destroyed by haemolysis is slow.Malaria and anaemia were observed to increase as the age of the pregnancy (Trimester) increased. The occurrence was least in the first trimester (29.0% and 26.0%) and highest in the third trimester (60.9% and 38.2%). The difference observed in the prevalence of malaria and anaemia within the trimesters was found to be statistically significant. This is consistent with the findings of Achidi et al. (2005) and Ouma et al. (2007) from similar studies in Cameroon and Western Kenya respectively. Prevalence of malaria has been reported to be at its peak in the second trimester up to the beginning of the third trimester. Dicko et al. (2003) however reported that malaria and anaemia are more common in the first and second trimester of pregnancy than in the third trimester. This present study also compared the haemoglobin level in malaria infected pregnant women with that of those not infected with malaria. The mean haemoglobin level of pregnant women infected with malaria was found to be lower than those of pregnant women not infected with malaria. This agrees with the findings of Erhabor et al. (2010) and Achidi et al. (2005) who reported that mean haemoglobin level of pregnant women who were positive for malaria parasite were significantly lower than those who were free of malaria parasite. The burden of malaria and anaemia especially among pregnant women, underscores the importance of effective prevention, treatment and control measures that will stem the tide of the high prevalence recorded in this study and many others. This will significantly reduce morbidity and mortality among pregnant women and improve the overall health of women of child-bearing age.
5. Conclusions
- This present study reveals a high prevalence of malaria among pregnant women of young and very old age and those who were carrying their first pregnancy. The study also observed a significant association between malaria infection and anaemia in pregnant women. Malaria and anaemia were also observed to be trimester and parity related. The high incidence of anaemia observed among Plasmodium falciparum parasitized pregnant women in this study underscores the need for regular malaria chemoprophylaxis and increased awareness for all pregnant women particularly in malaria endemic countries. A strengthening of existing control measures and the use of integrated approaches hold a great promise in solving the problem of malaria in pregnant women.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML