-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2017; 7(5): 211-215
doi:10.5923/j.ajmms.20170705.01

Clinical-Morphological Parameters of Tumor Tissue of Breast Cancer with Positive and Negative Phenotypes of Expression of Receptors to Estrogen and Progesterone
A. Kh. Rakhmanov1, A. A. Abduvaliev1, Z. Z. Khakimov1, Sh. R. Mavlanov1, F. W. Wang2
1Tashkent Medical Academy, Tashkent, Uzbekistan
2Chengdu Institute of Biology, Chinese Academy of Sciences, China
Correspondence to: A. Kh. Rakhmanov, Tashkent Medical Academy, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

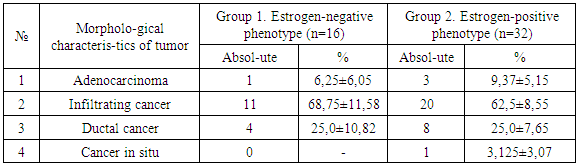
A study of clinical and morphological parameters of tumor tissue of breast cancer with positive and negative phenotypes of receptor expression to estrogens and progesterone (ER (-) / PgR (-)) was conducted. 48 samples of tumor tissue of breast cancer were selected for the study in patients who underwent surgery for the treatment of this disease at the Republican Cancer Research Center in Tashkent (Uzbekistan). The average age of women were 52,04±1,52 years old. There was positive expression to estrogen receptors (from 2+ to 3+ by 4 point scale count of immunohistochemical reaction) in 66,66±6,80% examination of tumor samples and the expression of receptors to estrogens were absent in 33,4±6,80% samples. There was positive expression to progestrone receptors (from 2+ to 3+ by 4 point scale count of immunohistochemical reaction) in 60,41±7,05% examination of tumor samples and the expression of receptors to progesterone were absent in 39,58±7,05% samples.
Keywords: Breast cancer, Expression of receptor, Estrogen, Progesterone
Cite this paper: A. Kh. Rakhmanov, A. A. Abduvaliev, Z. Z. Khakimov, Sh. R. Mavlanov, F. W. Wang, Clinical-Morphological Parameters of Tumor Tissue of Breast Cancer with Positive and Negative Phenotypes of Expression of Receptors to Estrogen and Progesterone, American Journal of Medicine and Medical Sciences, Vol. 7 No. 5, 2017, pp. 211-215. doi: 10.5923/j.ajmms.20170705.01.
Article Outline
1. Introduction
- Microchipping of the tumor cells’ DNA of breast cancer (BC) has made it possible to identify several genetically different forms of this disease. This molecular genetic classification has been actively used in clinical practice for the purpose of individualizing therapeutic approaches and studying new methods of treatment. According to the molecular genetic classification, the following variants of breast cancer are defined, which differ according to the prognosis and sensitivity to different types of drug therapy [1, 2]:• luminal A ER (+) and / or PgR (+) / HER-2 / neu (-);• luminal B: ER (+) / and / or PgR (+) / HER-2 / neu (+);• HER-2 / neu (+): ER (-) / PgR (-) / HER-2 / neu (+);• basal-like: ER (-) / PgRQ / HER-2 / neuQ.Histogenetic basal-like BC is associated with basal epithelium, which in the healthy mammary gland composes external layer adjacent to the basal membrane, lining the ducts and lobules. This is a morphologically and immunophenotypically heterogeneous population with the features of epithelial and smooth muscle cells, which is reflected in their name – myoepithelial [3, 4].In addition to other markers, the expression of high molecular basal cytokeratins (CK5/6, CK14, CK17), EGFR (HER1), p-cadherin, CAV1 and CAV2 are typical for these cells [5]. The expression of genes typical for basal /myoepithelial cells are also determined in the basal-like breast cancer cells. Many products of these genes perform a structural role, participate in the proliferation of cells, the oppression of apoptosis, migration and/or invasion i.e. in processes inherent for cancer [6]. At the same time, the expression of ER, ER-dependent and other genes characteristic for luminal epithelial cells of normal breast tissue, as well as HER-2 amplicon in basal-like tumors, is minimal [7]. Thus, at the base of the aggressive phenotype of basal-like tumors lies the corresponding genotype, indicating the origin from the least differentiated (possibly even stem) cells [8].Endocrine therapy is indicated for most patients with breast cancer (BC) with receptor-positive tumors (ER+/PR+) [9, 10]. Ovarian steroid production decreases with age and development of estrogen in postmenopausal women occurs primarily in peripheral tissues such as adipose tissue and adrenal androstenedione where in the corticosteroid is converted (transformed) first into estrone and then to estradiol. This peripheral conversion occurs via aromatase (enzyme complex consisting of the cytochrome P450 and flavoproteins), which is a catalyst for the converting of androgens to estrogens [11].Currently, the treatment of basal-like breast cancer with a negative phenotype of the expression of receptors for estrogens and progesterone (ER (-) / PgR (-)) is an actual problem in oncology. We conducted a study of clinical and morphological parameters of tumor tissue of breast cancer with positive and negative phenotypes of receptor expression for estrogens and progesterone (ER (-) / PgR (-)).
2. Material and Methods
- 48 samples of tumor tissue of breast cancer were selected for the study in patients who underwent surgery for the treatment of this disease at the Republican Cancer Research Center in Tashkent (Uzbekistan). Criteria for selection were a verified diagnosis of breast cancer and surgical intervention in this regard.The routine clinic examinations were carried out in all patients (biochemical status, clinical characteristics of the tumor, histological, ultrasound, radiographic examination, computer tomography).Determination of the expression of receptors to estrogens and progesterone was carried out by using test kits (DAKO) for the immunohistochemical study. The received results were subjected to the statistic processing with the using of standard software package Biostat 2009 on well-known method of variation statistics with an estimation of the statistic significance of indicators (M±m) and differences between groups were analyzed using the Student’s t-test. P<0.05 was considered significant.
3. Results and Discussion
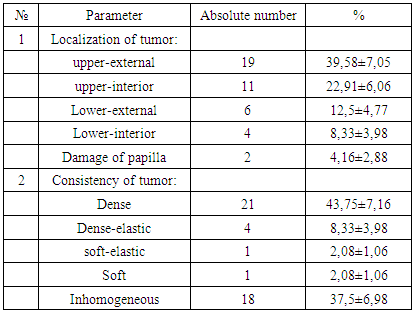
- The average age of women were 52,04±1,52 years old. The primary symptoms of women who addressed to medical care with the reason of breast disease and concomitant disease were given in 1st figure. It was determined that there was hereditary pathology i.e. “cancer family” where close relatives by female line suffered from the oncopathology of breast and reproductive organs length several generation in one woman (2,08±0,2%). The results of determining of localization and consistency of tumor were showed in table 1.
|
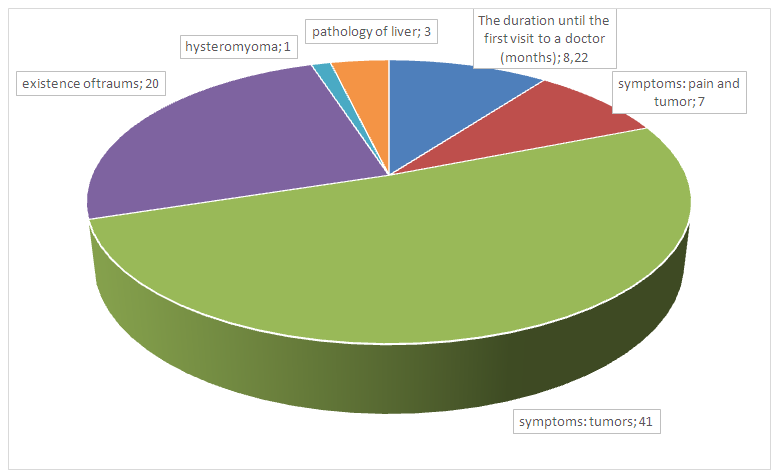 | Figure 1. The primary symptoms of women who addressed to medical care with the reason of breast disease and concomitant disease (in absolute numbers) |
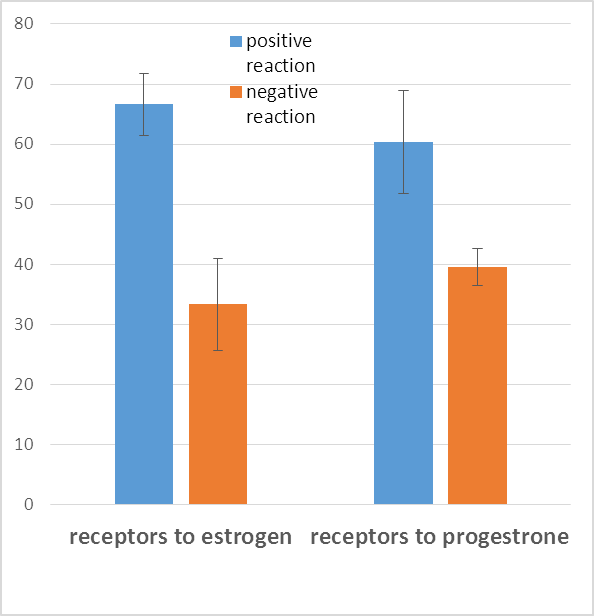 | Figure 2. The determination of expression of receptors to estrogens and progesterone on the surface of tumor cells with the immunohistochemical visualization method (n=48) |
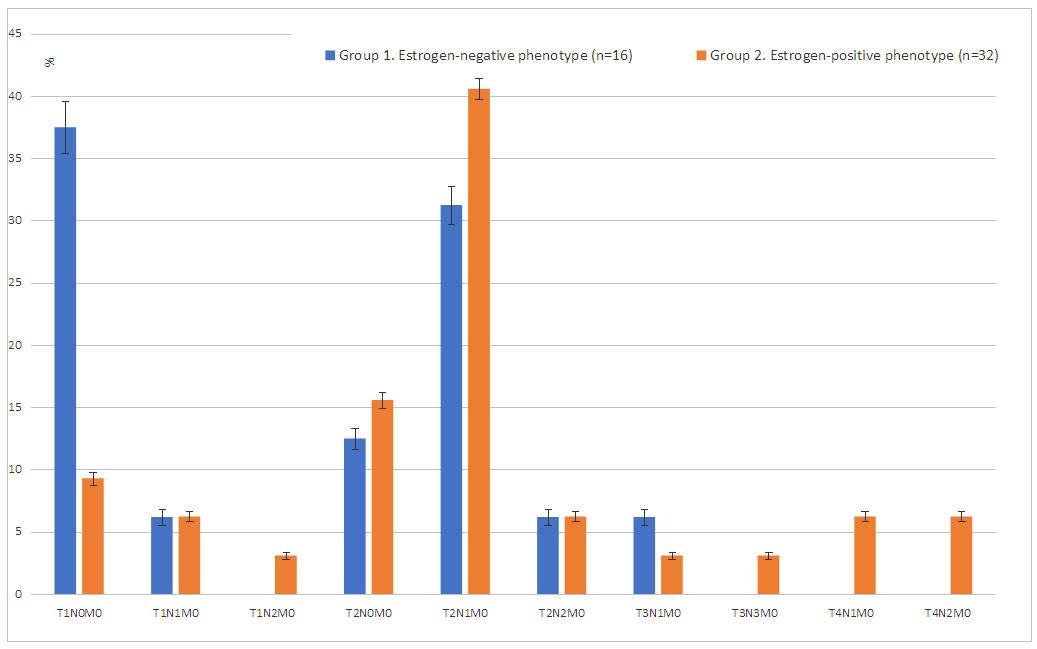 | Figure 3. The spread of tumor process by TNM system in patients with breast cancer with estrogen-negative and estrogen-positive phenotypes |
|
4. Conclusions
- In spite of bad prognosis, “negative” breast cancer is susceptible to the standard chemotherapy schemes including anthracyclin- and taxan-containing schemes. However, some patients do not reply to standard therapy. Knowledge on certain molecular characteristics of “negative” breast cancer helps to understand better the pathophysiology of disease and to develop more effective methods of treatment including the new generation of medicines consisting compounds from the natural resources.
ACKNOWLEDGEMENTS
- The part of this study was carried out by assisting Gildieva M.S., ScD, head of laboratory of tumor biology, Republican Scientific Center of Oncology of Uzbekistan.This scientific work was supported by the grant M/UZB-CHINA-28/2015 from Agency for Science and Technology of Uzbekistan.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML