-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2017; 7(4): 170-173
doi:10.5923/j.ajmms.20170704.02

Evaluation of Neuroendocrine Dysfunction in the Acute, Subacute and Chronic Phases of Traumatic Brain Injury
Ojieh G. C.1, Ebuehi O. A. T.2, Okhiai O.3
1Department of Medical Biochemistry, College of Medicine, Ambrose Alli University, Ekpoma, Nigeria
2Department of Biochemistry, College of Medicine, University of Lagos, Lagos, Nigeria
3Department of Nursing, College of Medicine, Ambrose Alli University, Ekpoma, Nigeria
Correspondence to: Ebuehi O. A. T., Department of Biochemistry, College of Medicine, University of Lagos, Lagos, Nigeria.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Traumatic Brain Injury (TBI) is a leading cause of premature death and disability worldwide. The greatest challenge associated with endocrine complications in individuals with TBI is early recognition of the subtle problems and in particular at what time after trauma the diagnosis should be made. The plasma testosterone and cortisol concentrations of established mild, moderate and severe TBI Patient were determined especially at the acute, sub-acute, and chronic phases of the TBI. Ninety six male adults without history of chronic ailment were enrolled in the study. In each case, blood was collected within 24h in the first instance, and subsequently at 1st week and 6th week of trauma. The plasma testosterone and cortisol levels were assayed using ELISA. Eighty five of the men with moderate to severe TBI had significantly low mean testosterone level of 3.8nmol/L (1.10ng /ml), moderate to severe TBI had lowered cortisol levels, while those with mild TBI had increased concentration in acute phase, but normal concentrations in the subacute and chronic phases of study. The testosterone and cortisol deficiencies may require replacement to improve recovery and healing from the effects of TBI.
Keywords: Traumatic brain injury (TBI), Testosterone, Cortisol, Endocrine dysfunction
Cite this paper: Ojieh G. C., Ebuehi O. A. T., Okhiai O., Evaluation of Neuroendocrine Dysfunction in the Acute, Subacute and Chronic Phases of Traumatic Brain Injury, American Journal of Medicine and Medical Sciences, Vol. 7 No. 4, 2017, pp. 170-173. doi: 10.5923/j.ajmms.20170704.02.
Article Outline
1. Introduction
- Traumatic Brain Injury (TBI) defined as a head trauma resulting in a brief loss of consciousness and/or alteration of mental state, is usually benign, but occasionally causes persistent and sometimes progressive symptoms [1]. The greatest challenge associated with endocrine complications in individuals with TBI is early recognition of the subtle problems and in particular at what time after trauma, the diagnosis should be made [1]. Traumatic brain injury (TBI) is a well-known public health problem around the world, and it is associated with increased morbidity, mortality and long-term disability [2]. TBI is an acute brain injury resulting from mechanical energy to the head from external physical forces. Operational criteria for clinical identification include; confusion or disorientation, loss of consciousness, post-traumatic amnesia, and other neurological abnormalities, such as focal neurological signs, seizure and/or intracranial lesion [1].The most commonly used criterion for classifying severity has been the Glasgow Coma Scale score. This is usually used for assessment when a person with suspected TBI presents to an Emergency Department or general practitioner. [3]. The mild, moderate and severe has a Glasgow coma scale score of 13–15, 9-12 and 3-8 respectively. A systematic review of the literature by the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury [4], concluded that a ‘true’ population-based rate of mild TBI would be more than 600 cases per 100,000 per year. ACC data suggest that for people with more severe TBI, approximately 50% were involved in a car crash. [5] This is in keeping with international data [4]. Cognitive deficits and other symptoms are common in the acute stage for those who have had a mild TBI. Most people have recovered fully by somewhere between three and 12 months following the injury. However, in both children and adults a minority will have longer-lasting effects of the TBI. There is also some evidence that adults who sustained a TBI in childhood or adolescence may have psychological impairments in adulthood [6].Neuroendocrine disorders (NED), primarily hypopituitarism, was first diagnosed by the German researcher Cyran in 1918 [7]. Until recently damage to the hypothalamus and pituitary gland following trauma was often not diagnosed until the post mortem examination [8]. The symptoms of NED include fatigue, insomnia, impaired cognition, memory loss, difficulty concentrating, and emotional and mood disturbances. [9, 10] Various combinations of these symptoms may occur and are similar to those of other post-mTBI conditions, such as sleep problems, post-concussive syndrome (PCS), memory and attention difficulties. Anterior pituitary deficiency (GH and Gonadotropin) account for the majority of chronic neuroendocrine disorders. The following ABI/TBI changes may be noted in hormones released by the pituitary gland. These hormones released include: (Adreno Corticotropic Hormone or Corticotropin (ACTH), Thyroid-Stimulating Hormone or Thyrotropin (TSH), Prolactin or Luteotropic hormone (LTH), Growth Hormone (GH), Follicle-Stimulating Hormone (FSH), Luteinizing Hormone (LH), Oxytocin, Testosterone, Estradiol, Antimullerian Hormone, Progesterone and so on [11]. Given that many of these deficiencies can be addressed medically, it is important to work with a physician for assessment and treatment of endocrine dysfunction when appropriate. Severity of TBI depends on many factors including the pre-injury condition of the brain, primary versus secondary pathophysiology of TBI, mechanisms of TBI and focal versus diffuse damage after TBI. [12]. Summarily, endocrine dysfunction is common after traumatic brain injury and since many of the signs of hormone deficiency are similar to the cognitive, behavioral, and somatic symptoms experienced after TBI, endocrine dysfunction may be easily overlooked.The objective of the present study sought to determine the plasma testosterone and cortisol levels in the acute, sub-acute and chronic phases of TBI, and to infer an imperative timely hormone replacement management or therapy.
2. Materials and Methods
- Ninety six male adults without history of chronic ailment were enrolled in the study. The level of consciousness of the patients was evaluated by GCS as soon as the patient was admitted and within 24 hours of injury. A score of 13-15 was considered mild, 9-12 moderate and 8 or less, severe TBI.
2.1. Blood Collection
- Blood was primarily taken from the enrolled TBI subjects. In each case, blood was taken within 24h in the first instance, and subsequently at 1st week and 6th week of trauma. The blood samples were collected by venopuncture in heparinized bottles and centrifuged. Plasma was extracted for testosterone and cortisol measurements, stored at -20°C until the samples were analyzed.
2.2. Plasma Hormonal Assay
- Plasma cortisol and testosterone levels were measured using radioimmunoassay by ELISA method. Quantitative assay of each plasma hormone was carried out using 25µl and following the prescription of Evans et al., [13]. The mean hormone level was recorded in milliliters (ml).
3. Results
- The prevalence of the neuroendocrine dysfunction at acute, sub-acute and chronic phases of TBI is presented in Table 1 and corresponding Figures 1 and 2, as mean plasma testosterone and cortisol levels for the mild, moderate and severe TBI at the three phases (acute, sub-acute and chronic respectively). The results obtained where compared with reference values of normal healthy adults.
|
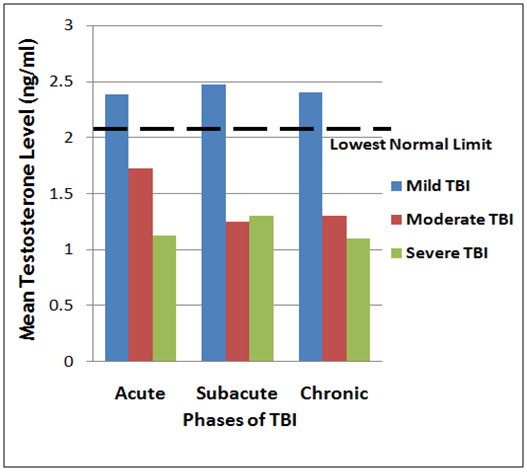 | Figure 1. Triple bar chart showing the variation of mean testosterone levels in the acute, sub acute and severe TBI groups |
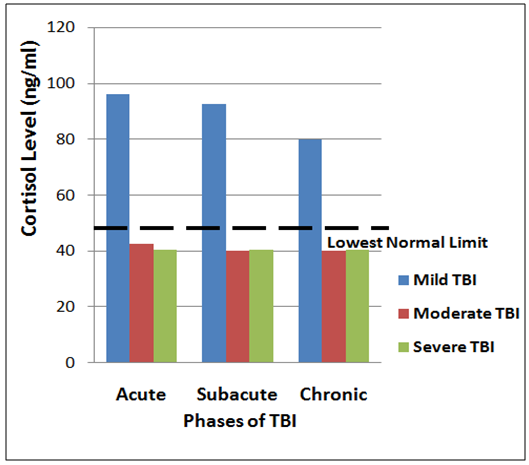 | Figure 2. Triple bar chart showing the variation of mean cortisol levels in the acute, sub acute and severe TBI groups |
4. Discussion
- Eighty five of the men with moderate to severe TBI had low mean plasma testosterone level of 3.8nmol/L (1.10ng/ml), with two of them that had burns reaching a Nadir of 1.2nmol/L (0.35ng/ml). The testosterone level became lower in one week and remained low in the six weeks of study. The remaining men with mild TBI had less lowering of testosterone level in 24h and 1 week which picked up towards normal in the 6th week of the subjects with moderate to severe TBI had lowered cortisol levels, while those with mild TBI had increased concentrations in acute phase, but normal concentrations in the subacute and chronic phases of study.The decrease in the plasma cortisol level with reference to the severity of the trauma is suggesting that increased severity of the trauma results in impaired HPA axis response. Severe trauma may give rise to more hypothalamic or pituitary damage, and results in blunted HPA-axis response to stress [6].Previous studies have clearly demonstrated that testosterone levels in males and estrogen levels in females significantly (p<0.01) decreased within the first 24h following TBI and remain lowered for 7–10 days [6, 9]. In this current study, mean plasma testosterone concentration has been shown to be significantly reduced with the severity of injury compared to the lowest limit of healthy individual. [14, 15]. In addition, it is also consistent with the findings of previous studies as mean total testosterone level in male patients was significantly lower in the moderate to severe TBI group when compared to the mild groups [9, 16]. The decrease in plasma cortisol level across the group probably suggest that cortisol might be a contributing factor to pituitary dysfunction, particularly in severe TBI patients. The progressive decline of plasma testosterone and cortisol levels provide a rationale behind reported improved neurological outcomes in TBI patients following acute GH and sex hormone replacement after TBI [15, 17].
5. Conclusions
- Data from this study further lend credence to the report that TBI may cause abnormalities of testosterone and cortisol secretions at the pituitary gland. The testosterone and cortisol deficiencies may require replacement to improve recovery and healing from the effects of TBI.
ACKNOWLEDGEMENTS
- This research article is dedicated to the memory of Dr. Godwin Chucks OJIEH (1962-2016), who died on January 18, 2016. The findings of this article were extracted from his PhD Biochemistry degree research work which he was doing under the supervision of Prof. O.A.T. Ebuehi, in the Department of Biochemistry, College of Medicine, University of Lagos, Lagos, Nigeria. The authors are grateful to the Management and Staff of Irrua Specialist Hospital, Irrua, Edo State, Nigeria, for the use of their facilities and patients for the study.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML