-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2017; 7(4): 165-169
doi:10.5923/j.ajmms.20170704.01

Biochemical Effect of Anti-Hypertensive Drugs and Vitamin C in Non-Hypertensive Albino Wistar Rats
Arit J. Ekpo1, Innocent A. Edagha2, Emmanuel I. Akpaneka1
1Department of Biochemistry, Faculty of Basic Medical Sciences, University of Uyo, Nigeria
2Department of Anatomy, Faculty of Basic Medical Sciences, University of Uyo, Nigeria
Correspondence to: Arit J. Ekpo, Department of Biochemistry, Faculty of Basic Medical Sciences, University of Uyo, Nigeria.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

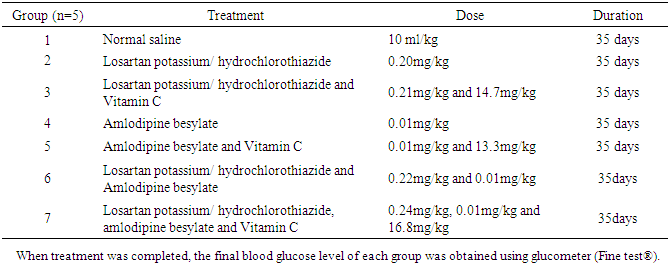
The combination effect of anti-hypertensive and antioxidant drugs; Losartan potassium/hydrochlorothiazide 100 mg/25 mg, Amlodipine besylate 5 mg, and Vitamin C 100 mg on blood glucose level and haematological indices in non-hypertensive female albino Wistar rats were investigated. Thirty-five (35) rats weighing (110-180 g) were divided into seven groups; group 1 received normal saline 10ml per kg body weight; group 2 received potassium/hydrochlorothiazide 0.20mg/kg body weight; group 3 received 0.21 mg and 14.7 mg of Losartan potassium/hydrochlorothiazide and Vitamin C respectively per kg body weight; group 4 received 0.01 mg of Amlodipine besylate per kg body weight; group 5 received 0.01 mg of Amlodipine besylate and 13.3mg of Vitamin C per kg body weight; group 6 received 0.22 mg of Losartan potassium/hydrochlorothiazide and 0.01 mg of Amlodipine besylate, per kg body weight; group 7 received 0.24 mg of Losartan potassium/hydrochlorothiazide, 0.01 mg of Amlodipine besylate and 16.8 mg of Vitamin C per kg body weight. Drugs were administered orally via oro-gavage needle. Serial blood glucose was obtained at week 1, week 3 and week 5 respectively. After 35 days, rats were sacrificed by chloroform inhalation, blood collected and analyzed. Results indicate that there was statistically significant increase in blood glucose estimation of test groups 2-7 compared to group 1; likewise there was statistically significant increase in white blood cells in group 2 and 3, increase in red blood cells in group 1, increase in haemoglobin in group 1, and increase in packed cell volume in group 1, whereas platelet was significantly unchanged. In conclusion, antihypertensive drugs mildly increase blood glucose levels and alter haematological parameters in albino Wistar rats, and may be suggestive likewise in human patients taking the drugs for prolong period.
Keywords: Losartan potassium/hydrochlorothiazide, Amlodipine besylate, Vitamin C, Blood glucose, Haematology
Cite this paper: Arit J. Ekpo, Innocent A. Edagha, Emmanuel I. Akpaneka, Biochemical Effect of Anti-Hypertensive Drugs and Vitamin C in Non-Hypertensive Albino Wistar Rats, American Journal of Medicine and Medical Sciences, Vol. 7 No. 4, 2017, pp. 165-169. doi: 10.5923/j.ajmms.20170704.01.
Article Outline
1. Introduction
- Of WHO’s six regions, the African region has the highest prevalence of hypertension estimated at 46% of adults aged 25 and above [1]. Hypertension is defined as systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg [2]. It is a long term medical condition in which the blood pressure in the arteries is persistently elevated. It is a non symptomatic disease condition with a long term effect on the vascular walls. Current drug therapy is based on a treatment algorithm in which drug classes are substituted or combined until blood pressure is brought under control [2]. Antihypertensive drugs are drugs that lower the arterial blood pressure which is the product of cardiac output and peripheral vascular resistance. These drugs may reduce the cardiac output by either inhibiting myocardial contractility or decreasing ventricular filling pressure by actions on the venous tone or blood volume via renal effect [3]. Ascorbic acid also known as vitamin C plays an important role due to its antioxidant capacity since it contributes to the maintenance of the vascular system and the reduction of atherogenesis through the production of prostacyclin and nitric oxide [4]. Vitamin C is considered a very strong reductant and radical scavenger; it reduces unstable oxygen, nitrogen, and sulfur radicals, and in addition, it acts as primary defense against aqueous radicals in blood [5], and pharmacokinetics and pharmacodynamics of drug metabolism often generate free radicals. Hypertensive patients of African ancestry respond better to diuretics and calcium blockers due to environmental, pharmacokinetic and pharmacodynamic factors that may contribute to the differential clinical response [6]. Angiotensin-converting enzyme (ACE) inhibitors are also known to induce less blood pressure lowering in patients of African than European Ancestry [7]. Indeed drug therapy can be rarely discontinued after blood pressure is brought back to normal range. Frequently, the drug dosage can be reduced with time. This reduction is important because antihypertensive drugs can produce uncomfortable side effect whereas hypertension itself may not produce uncomfortable symptoms, a situation that make patient compliance with the drug therapy difficult [8].The beneficial effect of the combination of Hyzaar (Losartan potassium/ hydrochlorothiazide 100mg/25mg) and Norvasc (Amlodipine besylate 5mg) for lowering blood pressure in spontaneously hypertensive rats has been examined [9]. Several meta-analysis of community-based hypertension study show that males slightly have higher rates of hypertension than females [10], although females where more consistent in adhering to treatment. This study aims at determining the combination effect of antihypertensive drugs Hyzaar (Losartan potassium/hydrochlorothiazide 100mg/25mg), Norvasc (Amlodipine besylate 5mg) and with Vitamin C on blood glucose level and haematological indices of non-hypertensive female albino Wistar rats.
2. Materials and Method
2.1. Chemicals and Reagents
- Amlodipine besylate (5 mg), Losartan potassium/ hydrochlorothoazide (100 mg/ 25 mg), Vitamin C (100 mg) all purchased from Lincoln Care Drug, Bronx, NY 10456. Chloroform purchased from Core Biomedicals Uyo, Akwa Ibom State. All chemicals and reagent were at analytical grades.
|
2.2. Experimental Animals
- Thirty five (35) female albino Wistar rats weighing 110-180g were used for this study, and they were obtained from the Department of Pharmacology and Toxicology, Faculty of Pharmacy, University of Uyo. These animals were allowed one week acclimatization after which they were reweighed and housed in cages under controlled environmental conditions of normal room temperature, relative humidity and a 12 hour light and dark cycle. The animals were adequately ventilated and the animals maintained regularly on the commercial rat chow. Tap water and food were provided ad libitum throughout the experimental periods. All procedures involving animals in this study conformed to the guide for the care and use of laboratory animals [11] and granted approval by the Department of Anatomy ethical committee, University of Uyo.
2.3. Preparation of Stock Solution
- One tablet of Losartan potassium (100 mg)/ hydrochlorothiazide (25 mg) was grounded into a clean beaker and was diluted with 10 mls of distilled water and was mixed by slightly shaking. The same procedure was repeated for Amlodipine besylate (5mg), and for Vitamin C containing 100 mg/tablet. Three (3) of the drugs were grounded, poured into a clean beaker, then diluted with 20 ml of distilled water, while the beakers containing each drug were labeled.
2.4. Preparation of Standard Solution
- 1 ml each of Losartan potassium/hydrochlorothiazide and Amlodipine besylate, was diluted with 9 ml distilled water in a labeled tube accordingly. The content was mixed by slightly shaking before administering the drugs to the rats based on their respective body weight. The drugs were administered orally through an oral cannula, and new stock and standard solution were prepared daily for administration.
2.5. Blood Glucose Estimation
- Blood glucose level was determined using glucometer (Fine test®) by capillary action of a drop of rat tail blood via needle prick on the test strip, and the blood glucose reading displayed on the glucometer. The blood glucose level was checked thrice during the course of the work on the first, third and fifth week respectively.
2.6. Animal Sacrifice
- At the end of the administration (35 days), the animals were fasted overnight (12 hrs), and anaesthetized by chloroform inhalation in a transparent desiccator. Blood samples were collected through cardiac puncture with 2 ml syringe into sterile plain tube containing anticoagulant (EDTA bottles) for plasma preparation which was used for the whole blood analysis.
2.7. Haematological Estimation
- This was done using Mythic 18 automated Haematology Analyser Model KX-21N, produced by Sysmex Company Japan. The parameters analyzed included Red blood cells (RBCs), White blood cells (WBCs), Haemoglobin (HB), Packed cell volume (PCV), Mean corpuscular volume (MCV), Mean corpuscular haemoglobin (MCH) and Platelets (PLT).
3. Results
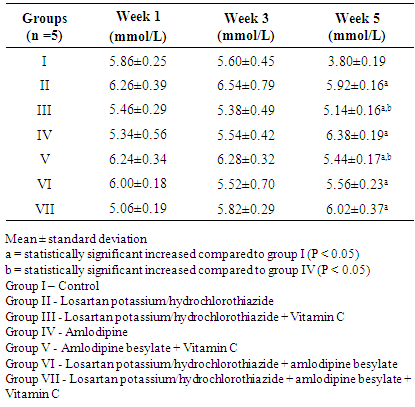
- The effect of Losartan potassium/hydrochlorothiazide, Amlodipine besylate and Vitamin C on blood glucose level of female albino Wistar rats was determined and the result is presented in Table 2. The blood glucose level of rats in groups I to VII was not statistically significant in the first and third week. In the fifth week, the blood glucose levels of group II to VII were significantly (P < 0.05) increased compared to group 1 (control). Also, blood glucose levels of group III and IV were significantly (P < 0.05) decreased compared group IV.
|
|
4. Discussion
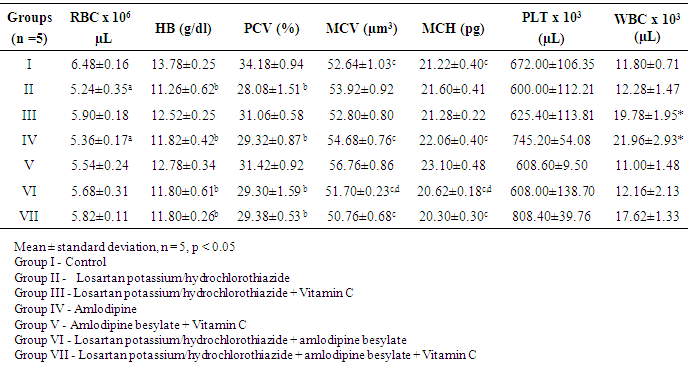
- Hypertension is a worldwide problem that affects approximately 15-20% of all adults [12]. The result of this study indicates that Losartan potassium/hydrochlorothiazide and Vitamin C maintains the blood glucose level within a specific normal range as well as with administration of Amlodipine with vitamin C. This is due to the antioxidant effect of Vitamin C which helps in mopping free radicals generated as a result of metabolism of these drugs. As shown in Table 2, the elevation of blood glucose level in groups II and IV administered Losartan potassium/ hydrochlorothiazide and Amlodipine respectively is consistent with the work of [13], who reported that administration of Losartan potassium/hydrochlorothiazide or Amlodipine causes hyperglycemia which could be reversed on withdrawal of treatment. In this study, elevation of blood glucose in groups administered with Losartan potassium/hydrochlorothiazide and Amlodipine respectively could be as a result of interference of these drugs with glucose metabolism as reported by [14]. It is observed that the blood glucose level could be maintained by addition of Vitamin C to monotherapy treatment with either Losartan potassium/hydrochlorothiazide or Amlodipine. It has been reported by [15], that thiazide diuretics promote hepatic insulin resistance, resulting in continued hepatic glucose production despite rising serum glucose or insulin levels. Rats that were administered Losartan potassium/hydrochlorothiazide and Amlodipine besylate had a reduced blood glucose level compared to rats that received monotherapy administration. This implies that the combine administration of Losartan potassium/hydrochlorothiazide and Amlodipine could also help maintain the blood glucose level within normal range. As presented in Table 3, this study also recorded some alterations in the haematological indices in the course of treatment. The decline in the test groups compared to the control of blood parameters; RBC, HB, PCV, MCV and MCH is indicative of anemia [16]. The RBC of rats in groups II and IV were relatively decreased compared to group I. Rats in groups II, IV VI and VII all had a decreased HB and PCV compared to group 1. The MCV of rats in groups I, III, IV, VI and VII were drastically reduced compared to group V. The MCH of rats in groups I, II, III, IV and VI were reduced compared to group V. The result thus obtained shows a decrease in RBC, HB, PCV, MCV and MCH in rats administered with Amlodipine. This decrease is an indicative of microcytic anemia which could be as a result of interference of Amlodipine with iron adsorption. The RBC, HB, PCV and MCH of rats administered with Losartan potassium/hydrochlorothiazide shows a significant decrease. This is an indicative of microcytic anemia which is consistent with the work of [17], which reported that administration of either Losartan potassium/ hydrochlorothiazide or Amlodipine as a monotherapy leads to hemolytic anemia. From the result obtained, it can be observed that groups administered Losartan potassium/ hydrochlorothiazide and Amlodipine could lead to anemia, this indicates the interference of these drugs in haematopoiesis. Likewise in group VI, rats administered with combination therapy of Losartan potassium/hydrochlorothiazide and Amlodipine besylate all had statistically decreased (p<0.05) HB, PCV, MCV and MCH which is an indicative of microcytic anemia caused as a result of iron deficiency. However, rats administered with Losartan potassium/ hydrochlorothiazide and Vitamin C or Amlodipine besylate and Vitamin C all had normal haematological indices compared to the control. This suggest that Vitamin C which is an antioxidant helps to mop up free radicals generated during metabolism and help cushion the effect of hemolysis.There was a non-significant (P > 0.05) outcome for platelets (PLT) in all the groups. Therefore they were no significant effect of Losartan potassium/ hydrochlorothiazide, Amlodipine besylate and Vitamin C on the platelet count of female albino Wistar rats.
5. Conclusions
- Administration of Losartan potassium/ hydrochlorothiazide and Amlodipine besylate therapy alters blood glucose level and haematological indices in non-hypertensive female Wistar rats. Long-term monotherapy with either Amlodipine besylate or Losartan potassium/hydrochlorothiazide could lead to hyperglycemia and hemolytic anemia, but the blood glucose levels and haematological composition can be maintained with the addition of Vitamin C to Losartan potassium/ hydrochlorothiazide or Amlodipine besylate to attenuate the antagonistic effect of the drugs.
ACKNOWLEDGEMENTS
- We appreciate the technical staff at the Laboratory Unit of the University of Uyo Medical Centre.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML