-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2017; 7(2): 79-86
doi:10.5923/j.ajmms.20170702.07

Cognitive Functions and Childhood Epilepsy with Centro-Temporal Spikes (BCECTS)
Abd El Magid M. Bayomi1, Ahmed Saad Elshemy1, Eman Fathalla Gad2, Islam Shabaan3
1Department of Pediatrics, Al-Azhar University, Assiut, Egypt
2Department of Pediatrics, Assiut University, Egypt
3Department of Psychiatry, Al-Azhar University, Assiut, Egypt
Correspondence to: Abd El Magid M. Bayomi, Department of Pediatrics, Al-Azhar University, Assiut, Egypt.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

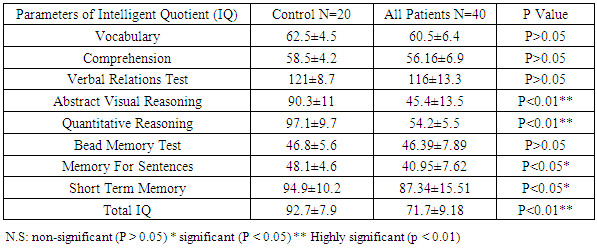
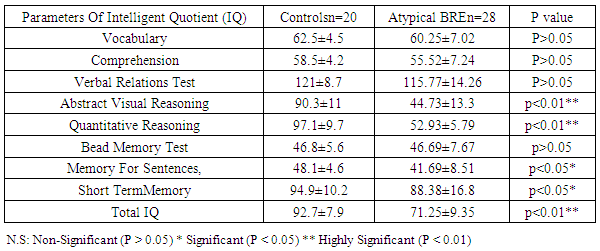
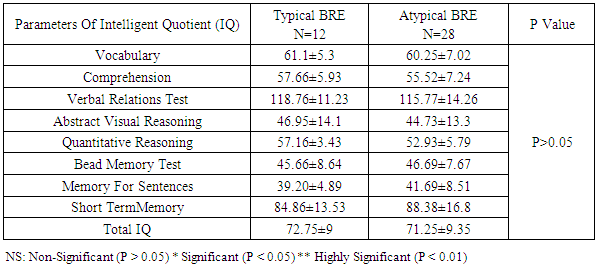
Benign childhood epilepsy with Centro-temporal spikes (BCECTS) or benign rolandic epilepsy (BRE) is the most common partial epilepsy syndrome in pediatric patients. This study aimed to study if there is any impairment in cognitive functions in the 2 variants of BCECTS. Patients and methods: The study included forty children with BRE according to the International League against Epilepsy classification (ILAE 1989), aged from 3 to 15 years (26 males and 14 females) in addition to 20 apparently healthy children as controls. All patients and controls were subjected to full and carful history, complete clinical and full neurological examination, EEG, Stanford Binet Intelligence Scale, fourth edition (SB4) and ADHD scoring system. Patients with single fit received no treatment, while those with recurrent fits received anti-epileptic drugs (AEDs). In addition, patients with cognitive and/or behavioral impairment received behavioral therapy. EEG, SB4 and ADHD scoring system were repeated 6 months after the last fit or initiation or change of AEDs to assess the efficacy and effect of therapeutic regimen on different patients. Results: sixty five % of patients with BRE were males and 35% were females. The mean ±SD of age was 9±2.33. Fifty seven and half % of our patients with BRE were coming from rural areas, and only 35% had positive family history of epilepsy. All patients with typical BRE (n=12) ororo-facial nocturnal seizures with frequency of two fits in 20 out of 24 and single fit in 4 out of 24, their fits lasted in less than 5 minutes and in all of them the level of consciousness was preserved and the majority of them recovered completely on carbamazepine monotherapy, 4 of them were recovered completely without treatment. Patients with atypical form of BRE (n=28) showing generalized tonic clonic seizures and Hemi-convulsion and associated with loss of level of consciousness, 25 of them responding to carbamazepine monotherapy. Bilateral Centro-temporal spikes (CTSs) were found in 50% of patients and in 9 out of 12 patients with typical BRE but in only 11 out of 28 patients with atypical BRE. A statistically significant decrement in total IQ and increment in ADHD scoring system in all patients compared to controls. A statistically significant negative correlations were found between total IQ and inattention in all patients and patients with atypical BRE respectively (r=-0.077 & r=-0.072). A statistically significant positive correlations were found between total IQ and hyperactivity in all patients and patients with atypical BRE respectively (r=0.099 & r=0.154). patients showed only clinical improvement within 6 month therapy , however no statistically significant differences were observed in our patients with BRE prior and 6 month after therapy in EEG, SB4 and ADHD scoring system (p>0.05). Conclusions: Characteristic features of seizures of typical BREare oro-facial nocturnal seizures recovered completely with or without treatment. Characteristic features of seizures of atypical BRE are recurrent generalized tonic clonic seizures and hemi-convulsions, associated with loss of level of consciousness and some of them required combined AEDs. Centro temporal spikes (CTSs) are the most prominent EEG findings in patients with typical and atypical BRE. A statistically significant decrement in total IQ was found in all patients compared to controls. BRE might show an obvious and prominent diverse effect on attention and activity in our patients with a less pronounced effect upon impulsivity.
Keywords: BRE, a typical BRE, CTSs, IQ, ADHD
Cite this paper: Abd El Magid M. Bayomi, Ahmed Saad Elshemy, Eman Fathalla Gad, Islam Shabaan, Cognitive Functions and Childhood Epilepsy with Centro-Temporal Spikes (BCECTS), American Journal of Medicine and Medical Sciences, Vol. 7 No. 2, 2017, pp. 79-86. doi: 10.5923/j.ajmms.20170702.07.
1. Introduction
- Benign childhood epilepsy with Centro-temporal spikes (BCECTS) or benign rolandic epilepsy (BRE) is the most common partial epilepsy syndrome in pediatric patients. Benign childhood epilepsy with Centro-temporal spikes characteristically starts between 3 and 10 years of age, with a peak age of onset at 9 years, and complete resolution after 16 years [1].A strong genetic susceptibility has been suggested in BCECTS. Very recently, a genome-wide study demonstrated linkage of the Centro-temporal sharp wave Electroencephalogram (EEG) trait to 11p13 and several polymorphic markers in the Elongated Protein Complex 4 (ELP4) gene showed association with the BCECTS phenotype [2]. BCECTS, are usually brief, unilateral, tonic, clonic, or tonic-clonic, convulsions involving the face, lips, tongue, pharyngeal, and laryngeal muscles, with speech arrest, saliva drooling without loss of consciousness. Classically the seizures occurs during sleep, usually shortly after falling asleep or before awake, the seizures often benign focally with clonic movements of the mouth, guttural sounds, increase salivation and strange sensation of the tongue, it may become secondary generalized. The most common inter-ictal EEG features are Centro-temporal diphasic or triphasic sharps or spikes occasionally spreading to the homologous contra-lateral region. BCECTS usually is associated with “idiopathic” conditions but it has also been reported with brain lesions such as cortical dysplasia [3].Few ictal EEG in cases of rolandic epilepsy have been recorded, in these cases the seizures begin with fast rhythms and spikes in the rolandic area that is contralateral to the clinical seizures, with a progressive increase in amplitude and admixture of slow or polyspikes waves [4]. Although BCECTS is considered a benign form of childhood epilepsy that occurs in children who show normal mental development, recent researches support the view that children with BCECTS show deficient performance in various neuropsychological areas, without a definition of a uniform profile [5]. On the other hand, the impact of epilepsy on cognitive function is complex, with many variables that can influence cognitive ability and interact, making it is difficult to determine which factors contribute to the cognitive impairment [6]. In children with rolandic epilepsy, recently clearly indicate an impairment in selectivity (impulsivity, focused attention, selective attention, aspects of divided attention) and in one measure of intensity (arousal) of attention whereas, the other measure of intensity of attention (vigilance) showed noimpairment. Moreover, deficits of speech-related abilities seemed to be independent of the effects of antiepileptic treatment and are reversible after remission of epilepsy [5].Comorbidity between BCECTS and altered development of speech motor control (also known as speech sound disorder) has also been further demonstrated in a recent case-control study [7]. Aim of the work: This study aimed to make comparison between various types of benign rolandic epilepsy (BRE) regarding to different clinical presentations, EEG results, types of cognitive and behavioral impairment and response to treatment. Also this study aimed to find any possible correlations between the different studied variables.
2. Subjects and Methods
- The study included forty children with benign rolandic epilepsy, aged from three to fifteen years (26 males and 14 females) in addition to twenty apparently healthy children of matched age, sex, and nutritional status as controls. The present work was conducted from February 2015 till the end of December 2015. The parents of all patients and controls give a written consent form for agreeing their children to participate in the study. The work has been approved by Al-Azhar Assiut University ethical committee.Inclusion criteria: Children ages ranged between 3 and 15 years and diagnosed as BRE according to the International League against Epilepsy classification [8].Exclusion criteria: children with epilepsy other than (BRE) or other conditions mimic epilepsy and Patients proved to have abnormalities on neuroimaging such as CT and MRI were excluded from our study. All patients and controls were subjected to the following: 1. Full and carful history was obtained from patients and their parents including age, sex, feeding pattern, detailed history of seizures including history of the first fit, frequency of fits, time of fit, prodroma, aura, ictus, post ictus, and precipitating factors, history of associated neurological complaint, past history of other system affection, family history of similar condition, history of drug intake and if the seizures were recurrent or first attack. Complete clinical and full neurological examination was done including blood pressure measurement and routine investigations including full blood count, blood glucose, serum calcium, potassium and sodium. Patients with single fit received no treatment, while those with recurrent fits received AEDs (carbamazepine, oxcarbamazepine, sodium valproate or combined therapy). In addition patients with cognitive and/or behavioral impairment received behavioral therapy. 2. Electroencephalogram (EEG). 3. Brain computerized tomography (CT) and magnetic resonance imaging (MRI). 4. The Stanford Binet Intelligence Scale, fourth edition (SB4), [9], Modified by Melika (1998), [10]. 5. Criteria for ADHD by (American Psychiatric Association, 2000), modified by Soheirkamil, 2010, [11]. The previous investigations apart from neuroimaging (EEG, SB4 and ADHD scoring system) were repeated 6 months after the last fit and initiation or change of AEDs to assess the efficacy and effect of therapeutic regimen on different patients.
3. Results
|
|
|
|
 | Figure 1. Correlation coefficient between total IQ and inattention in all patients with BRE |
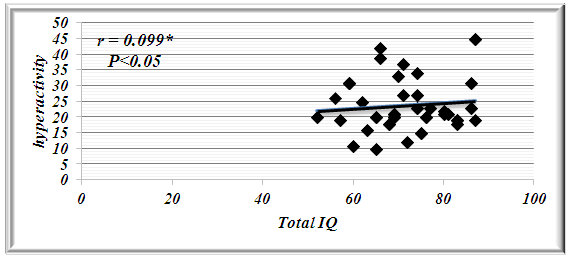 | Figure 2. Correlation Coefficient between total IQ and Hyperactivity in All Patients with BRE |
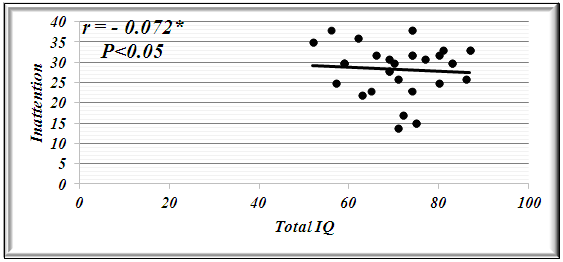 | Figure 3. Correlation Coefficient between total IQ and Inattention in Patients with Atypical BRE |
 | Figure 4. Correlation Coefficient between total IQ and Hyperactivity in Patients with Atypical BRE |
4. Discussion
- Benign rolandic epilepsy or benign childhood epilepsy with Centro-temporal spikes (BCECTS) is the most common epilepsy syndrome in childhood [12]. In the present study it was founded that 65% of patients with BRE were males and 35% were females our results of male predominance were in agreement with results of Wirrell 1998, [13] who stated that BRE is more common in males with a ratio of approximately 3:2 also Larsson and Eeg-Olofsson 2006, [14] reported that there was 1.5 male predominance in BRE. The mean ±SD of age was 9±2.33 in our patients with BRE and showed no significant differences from controls (8.5±2.84). Chahine and Mikati 2006, [15] reported that most children will outgrow the syndrome BRE around the age of 3-13 years with a peak around 8–9 years and stops around age 14-18 years. Fifty seven % of our patients with BRE were coming from rural areas, and only 35% had positive family history of epilepsy, indeed Lerman 1992, [16] in his study reported that clinically, patients with BRE frequently have a family history of benign epilepsy. Febrile seizures are common (10–20%) before rolandic seizures [17]. Family history was strongly implicated in generalized epilepsy and research has recently found a number of genetically determined partial epilepsies, and partial epilepsies are often preceded by febrile seizures [18]. In this study, the age of onset of fits in the majority of patient (80%) and in all patients with typical BRE was 7-11years these results was in accordance with Larsson and Eeg-Olofsson (2006), [14] who stated that the age of onset ranges from 1 to 14 years with 75% starting between 7-10 years , also Lerman (1992), [16] reported that the onset occurs between the ages of 3 and 13 years, in 76 % between 5-10 years, with a peak at 9 years.The age of onset of fits in our patients with atypical BRE was 7-11 years in 50% of patients, and less than 7 years in 17.5% of patients, Kramer (2008), [12] stated that the early age at onset with in the features of atypical form of BRE. In the current study all patients with typical BRE (n=12) oro-facial nocturnal seizures with frequency of two fits in 10 out of 12 and single fit in 2 out of 12, their fits lasted in less than 5 minutes and in all of them the consciousness was preserved and the majority of them recovered completely on carbamazepine monotherapy, in addition to 2 out of 12 recovered completely without treatment. on the other hand patients with atypical form of BRE (n=28) generalized tonic clonic seizures and Hemi-convulsions in16 out of 28 and 7 out of 28 patients with atypical BRE respectively, the seizures are more frequent and lasted from 5-20 minutes and associated in all of them with loss of level of consciousness, 3out 28 resist to carbamazepine monotherapy, 3 out 28 not receiving treatment as their fits not recurred, the remaining 25 responding to carbamazepine monotherapy. The previous characteristics of seizures in our patients whether typical or atypical were found to be in agreement with many researchers, Arzimanoglou, et al; 2004, [19] who reported that the frequency of seizures in children with BECTS is usually low, with 25% of patients having a single episode and 50% having fewer than 5 fits. Panayiotopoulos and Chrysostomos, 2013, [20] concluded that the cardinal features of rolandic epilepsy are infrequent, often single, focal seizures consisting of unilateral facial sensorimotor symptoms (30% of patients), oro-pharyngolaryngeal manifestations (53% of patients), speech arrest (40% of patients), and, hypersalivation (30% of patients), Consciousness and recollection are fully retained in more than half (58%) of rolandic seizures, In the remainder (42%), consciousness becomes impaired during the ictal progress and in one third there is no recollection of ictal events. Neri, et al; 2012, [21] in their study stated that the total number of seizures in children with BRE is low, the majority of patients having fewer than 10 seizures, 10–20% have just a single seizure. About 10–20% may have frequent seizures, but these also remit with age and remission usually occurs within 2–4 years from onset and before the age of 16 years. Piccinelli, et al; 2008, [22] stated that seizures of BRE may be worse in children with onset of seizures before 8 years of age. Engel, 2006, [23] found that progression to hemi-convulsions or generalized tonic–clonic seizures occurs in around half of children and hemi-convulsions may be followed by postictal Todd’s hemi paresis. The diagnosis can be confirmed when the characteristic Centro- temporal spikes are seen on electroencephalography (EEG). Typically, high-voltage spikes followed by slow waves are seen. Given the nocturnal activity, a sleep EEG can often be helpful. Technically, the label "benign" can only be confirmed if the child's development continues to be normal during follow-up [15]. EEG abnormalities of BCECTS patients include: a negative sharp wave (>100μV) with a relatively blunted peak, followed by a prominent positive wave whose amplitude may be up to 50% of that of the preceding sharp wave [24]. In the present study bilateral Centro-temporal spikes (CTSs) were found in 50% of patients and in 9 out of 12 patients with typical BRE but in only 11 out of 28 patients with atypical BRE. Right Centro- temporal spikes (CTSs) were found in 20% of patients and in 1 out of 12 patients with typical BRE but in only 8 out of 28 patients with atypical BRE. Left Centro-temporal spikes (CTSs) were found in 10% of patients and in 1 out of 12 patients with typical BRE but in only 4 out of 28 patients with atypical BRE. Our results regarding EEG characteristics in our patients with typical and atypical BRE were found to be in accordance with Langill and Wrong 2003, [25] who reported that CTSs may be unilateral, but are more often bilateral, independently right or left. They are abundant (4–20/min) and usually occur in clusters. CTSs amplify during stages I–IV of sleep by a factor of two to five times without disturbing sleep organization. After sleep, the most common form of activation of CTSs (10–20%) is somatosensory stimulation, mainly of the fingers and toes. Lüders., et al., 1987 [26] found that the RDs are located unilaterally in around 60% and bilaterally in around 40% with the foci tending to shift from side to side asynchronously and synchronously. The remainder of EEG characteristics in our patients was in form of fronto-central spikes (FCSs), centro-parietal spikes (CPSs) and Centro-occipital spikes (COSs). These were rare findings and occurred in 2 out of 12 patients with typical BRE and in 6 out of 28 patients with atypical BRE. Kellaway 1981, [27] in his study reported that RD may be located in the Centro-parietal, fronto-central or Centro-occipital areas. Its localization in general is age dependent with parieto-occipital foci usually occurring before the age of four while Centro-temporal (mid-temporal) RD peak around the age of eight. Drury and Beydoun 1991, [28] found RD outside the Centro-temporal area in 21% of children with a typical history of RE and stated that the most important feature is the monomorphic show and not the location. Cognition in epilepsy is complex because problems may be intrinsic to the epilepsy itself and its underling neuropathology, seizures can affect cognition as caninter-ictal EEG activity, psychosocial and family problems. Also, although AEDs cansuppress/decrease seizures by suppressing epileptiform discharges, the way in which they do this can also interfere with cognitive functioning [29]. In the present study it was found that among the IQ parameters by SB4, total IQ, abstract visual reasoning, quantitative reasoning memory for sentences and short term memory showed a statistically significant decrement in all patients, patients with typical BRE and patients with atypical BRE when compared to controls. However the current study showed no statistically significant differences between patients and controls in the rest of IQ parameter which were vocabulary, comprehension, verbal Relations test, abstract visual reasoning and bead Memory test. Also no statistically significant differences were found between our patients with typical and atypical BRE in all IQ parameter. Our results regarding IQ parameters in patients and controls were found to be in agreement with many researchers. Neri, et al; 2012, [21] reported that children with rolandic seizures may develop usually mild and reversible linguistic, cognitive and behavioral abnormalities during the active phase of the disease. Also, Westerveld, (2010), [30] stated that neuro-cognitively, children with BRE have been shown to be at risk for a variety of neuropsychological deficits including deficits in overall intellectual functioning, memory, attention, and executive functioning. Bedoin et al; 2006, [31] suggest that a relationship exists between cognitive functions and CTS lateralization. Recalling the normal functioning of the brain, one would expect this be true, with CTS in the left and right hemisphere affecting left and right-brain functioning, respectively. For most people, the left hemisphere is the primary mediator of verbal functions, including reading and writing, understanding and speaking, verbal ideation, verbal memory, as well as the numerical symbol system [32]. School age children with epilepsy frequently present with comorbid Attention-Deficit/ Hyperactivity Disorder (ADHD). Estimates of the prevalence rates of ADHD in pediatric epilepsy suggest somewhere up to 40 percent of children with epilepsy also have ADHD which increases in children who require special education services. Children with comorbid epilepsy and ADHD appear to differ from community samples of children with ADHD in that they are more likely to have ADHD – predominantly inattentive type and are more likely to have an equal ratio of males to females [33]. In the present study moderate and severe inattention were found in 30% and 56.25% of patients with BRE respectively. Moderate inattention occurred in 8 out of 28 patients with atypical BRE but in only 4 out of 12 patients with typical BRE. Severe inattention occurred in 16 out of 28 patients with atypical BRE but in only 6 out of 12 patients with typical BRE. Moderate and severe hyperactivity were observed in 45% and 25% of our patients with BRE respectively. Moderate hyperactivity occurred in 5 out of 12 patients with typical BRE but in only 12 out of 28 patients with atypical BRE. On the other hand severe hyperactivity was found in 9 out of 28 patients with atypical BRE but in only 2 out of 12 patients with typical BRE. In our patients moderate to severe impulsivity was found in 30% of patients with BRE and in 11 out of 28 patients with atypical BRE but in only 2 out of 12 patients with typical BRE. We can conclude that BRE might show an obvious and prominent diverse effect on attention and activity in our patients with a less pronounced effect upon impulsivity. Despite of these effects on inattention and hyperactivity, no statistically significant differences were founded between our different studied patients with BRE (typical & atypical) as regard to ADHD scoring system. These results were found to be in accordance to different studies, Vega, et al; 2010 [33] and Killory, et al; 2011, [35], in their studies in children with both absence seizures and BRE founded attention difficulties in each of them. With respect to BRE, a recent review of literature in this area founded that all controlled studies demonstrated impairments in all tested attention networks, suggesting that there may be a more widespread functional cortical disturbance in BRE than previously thought [35]. Indeed, a recent study by Bennet-Back., et al 2011, [36] indicated that among 40 patients with “benign” epilepsy, 70 percent were diagnosed with ADHD as compared to a control group of siblings where only 17 percent were diagnosed with ADHD. Parisi et al 2010, [6] in their study stated that Symptoms of attention deficit hyperactivity disorder (ADHD) were more common in some particular types of epilepsies, such as rolandic epilepsy, representing a significant risk factor for academic underachievement. In our study statistically significant negative correlations were found between total IQ and inattention in all patients and patients with atypical BRE respectively (r=-0.077 & r=-0.072). In contrary statistically significant positive correlations were found between total IQ and hyperactivity in all patients and patients with atypical BRE respectively (r=0.099 & r=0.154). These results were supported by the results of Angela, 2010, [37] who reported that the child’s IQ’s must be considered as underestimates of his or her current intellectual functioning because of the behavioral interference, as extreme distractibility or anxiety can easily have some impact on the child’s score on any Wechsler Intelligence Scale for Children (WISC) subtest. In the present study, reassessment of the effect of 6 month therapy whether AEDs and/or behavioral therapy upon clinical , EEG , SB4 and ADHD scoring system, it was found that all patients showed only clinical improvement with no recurrence of fits within 6 month therapy. However no statistically significant differences were observed in our patients with BRE prior and 6 month after therapy in EEG, SB4 and ADHD scoring system (p>0.05). This might indicate that 6 month therapy were not sufficient to judge the effect on EEG, SB4 and ADHD scoring system. These results were found to be in agreement with Panayiotopoulos and Chrysostomos, 2013, [20] who stated that, the frequency, location and persistence of CTSs do not determine the clinical manifestations, severity and frequency of seizures or the prognosis.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML