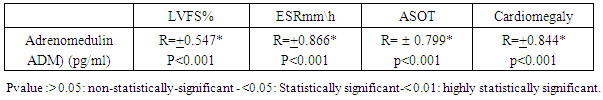-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2017; 7(2): 74-78
doi:10.5923/j.ajmms.20170702.06

Evaluation of Adrenomedullin Levels in Children with Acute Rheumatic Feverand Its Correlation to Left Ventricular Function
Hekma S. Farghaly1, Eman Fathalla Gad1, Alaa-Eldin A. Hassan2, Tahrasherif3, Madleen Adel A. Abdou3
1Pediatric Department, Assuit University, Assiut, Egypt
2Pediatric Department, Al-Azhar University, Assiut, Egypt
3Clinical Pathology, Assuit University, Assiut, Egypt
Correspondence to: Hekma S. Farghaly, Pediatric Department, Assuit University, Assiut, Egypt.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Acute rheumatic fever (ARF) is an inflammatory, autoimmune disease resulting from inadequate treated group A β-hemolytic streptococci (GAS) tonsilopharingitis. This disease commonly affect, heart, joints, skin, and brain. Carditis, arthritis, chorea, subcutaneous nodules, and erythema marginatum are the major manifestations. Fever, arthralgia, raised acute phase reactants (ESR and CRP), and prolonged PR interval on ECG are the minor manifestations of ARF [1]. Plasma adrenomedullin (ADM) levels are elevated in various pathological states including cardiovascular and inflammatory diseases [2]. We aim to evaluate ADM levels in children with acute and convalescent rheumatic fever (ARF). And its correlation to left ventricular function. Methods: This case -controlled prospective study included 45 patients with ARF (5-15 years) and 30 age/gender-matchedcontrol subjects. adrenomedullin levels were studied in the acute and convalescent phase of ARF. ESR, ASOT, CRP, CXR, ECG, ECHO are done for all patient and control subjecs, The study was carried out between januaary 2016 and jun 2016 in cardiology unit assuit University children Hospital. Results: All acute phase reactants (ESR, ASOT, CRP were significantly higher in studied cases than control). 22 cases 73.3% OF the cases have cardiomegaly, 26 cases 86.6% have prolonged PR interval, 23 cases 51.1% have mitral regurge 7 cases (15.5%) have mitral regurge and aoricregurge while 5 cases (11.15) have aortic regurge only. plasma ADM was significantly higher (p<0.05) in children with ARF, regardless of whether they were in acute or convalescent phase of disease. Plasma ADM levels were 74.43+3.4 pmol/mL in acute phases, 59.35+1.45 pmol/mL in the convalescent phase, and 44.79+3.12 pmol/mL in control group. The differences were statistically significant for all (p<0.05). Left ventricular fraction shortening (FS) was significantly low in the patients during acute phase than control. Plasma ADM levels in the acute phase of disease showed significant negative correlation with the left ventricular fraction shortening (FS) (r=-0.45, p<0.05). Conclusions: In patients with acute and convalescent rheumatic fever, ADM levels were high compared to those of healthy subjects and this could be used as a complementary tool in the treatment and prognosis of ARF.
Keywords: Adrenomedullin, Rheumatic fever, Left ventricular failure
Cite this paper: Hekma S. Farghaly, Eman Fathalla Gad, Alaa-Eldin A. Hassan, Tahrasherif, Madleen Adel A. Abdou, Evaluation of Adrenomedullin Levels in Children with Acute Rheumatic Feverand Its Correlation to Left Ventricular Function, American Journal of Medicine and Medical Sciences, Vol. 7 No. 2, 2017, pp. 74-78. doi: 10.5923/j.ajmms.20170702.06.
1. Introduction
- Although acute rheumatic fever (ARF) has declined in Europe and North America in incidence over the past 4 to 6 decades, the disease remains one of the most important causes of cardiovascular morbidity and mortality among socially and economically disadvantaged populations all over the world, especially in the developing countries that are home to the majority of the world’s population. Incidence rates in these countries still reach epidemic levels [3].Rheumatic fever (RF), a complication following an episode of group A streptococcal pharyngitis, is an acute, immunologically mediated, multisystem inflammatory disorder. Rheumatic fever is generally classified as a connective tissue disorder involving multiple organs [4]. The diagnostic criteria established in 1944 for RF was revised and updated by American Heart Association in 2015 [5]. RF occurs mostly in the young (5-15 yr) [6]. The pathogenic role of inflammatorycytokines, such as IL-8, IL-6, and alpha tumor necrosis (TNF-α) has been recognized [7]. Adrenomedullin (ADM), a hypotensivepeptide [8] has also been identified as a potent vasodilator. Indeed, ADM appears to play a pivotal role in both reprioritizing thebiological needs of tissues and organs during the three phases of inflammatory response as well as a role in restoring homeostatic equilibrium to the body [9]. It also plays a critical role in several diseases such as cancer, diabetes, cardiovascular andrenal disorders [9, 10].Many other studies have examined the possible role of AM in adults with chronic heart failure [11], hypertrophic cardiomyopathy, idiopathic dilated cardiomyopathy, and congestive heart failure [12]. The aim of the study was to assess the level of adrenomedullin in children with acute rheumatic fever as compared with healthy subjects, as well as to correlate the ADM values with left ventricular function during acute phases of acute rheumatic fever.
2. Patients and Methods
- The study included 45children with ARF (age range 5-15 years; mean 12±2 years, (21 boy and 24 girl) and 30 healthy, age and sex matched as control subjects. The study was carried out between January 2016 to June 2016 in the Department of pediatric cardiology of Assuit University children Hospital. Acute rheumatic fever was defined by two major, or one major and two minor manifestations accompanied with supporting evidence of antecedent group a streptococcal infection as a positive throat culture, or elevated or rising anti-streptolysin (ASO) titer and echocardiographic findings [9, 10].Patients were studied in acute and convalescent phases of the disease. The presence of new murmur of aortic or mitral regurgitation was considered as clinical evidence of carditis. This was confirmed by echocardiographic guidelines to define pathological mitraland aortic insufficiency [9, 10]. All patients had signs of inflammation in acute phase, clinically and laboratory such as tachycardia, fever, pallor, anorexia and elevated ASOtiter, erythrocyte sedimentation rate (ESR). They were treated with bed-rest, acetylsalicylic acid as an analgesic and antipyreticfor arthritis, penicillin, and corticosteroid for patients with severe carditis and the treatment continued until acute inflammation was subsided. We excluded Patients with congenital heart diseases with surgery, renal, hepatic diseases and hypertension, because they could affect plasma levels of ADM [13]. All subjects gave written informed consent before enrollment.Study designThe study, case-controlled and prospective, the blood samples for the acute phase were obtained within the first 48 h of diagnosis. The samples for the convalescent phase were obtained within 3 months), when all symptoms had completely disappeared. Only one blood sample was taken from each control subject. The samples were kept at -80°C until the study day. Blood samples were collected in tubes with 2mg/ml of ethylenediaminetetraacetic acid (EDTA)-2Na and 500 KIU/ml aprotinin. After collection, samples were promptly centrifuged at 1600 x g for 15 minutes at 4°C. Total plasma ADM was measured by using enzyme immunoassay kit (EIA) (Human Adrenomedullin). Phoenix Pharmaceuticals Inc. California, USA). The concentrations of plasma ADM (pmol/mL) were calculated from the standard curve for the known concentration of ADM.Statistical analysisValues are given as Mean Standard Deviation, Median (Range) and proportions/percentage. Normality was tested by Shapiro-Wilkmethod. Data were analyzed with Mann-Whitney U test, Wilcoxon signed rank test and Chi-square test based on statistical assumptions using Statistical Package for the Social USA). Pearson correlation was used to identify the relationship between the variables. All p values <0.05 were considered statistically significant.
3. Results
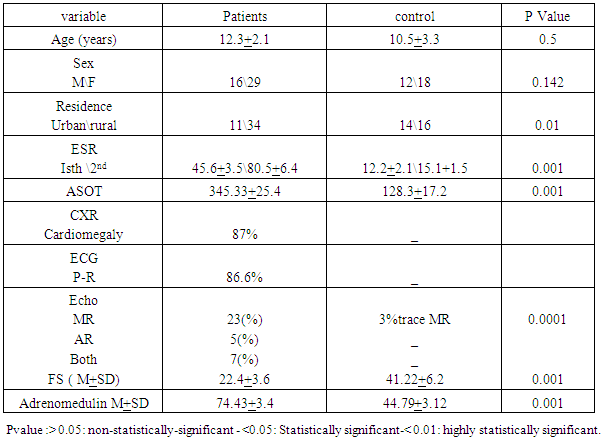
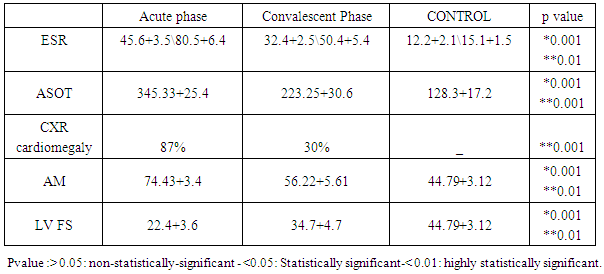
- Table 1 shows that rheumatic patients (n=45) and control were comparable as regard age, gender and residence. Acute phase reactants (ESR, ASOT and CRP) are significantly higher in patients than control .mean LVFS was significantly lower in patients than controls (p>0.001). Adrenomedulin was significantly higher in patients than control (p>0.001).Table 2 shows that acute phase reactants (ESR & ASOT and CRP) were significantly higher in patients during acute phase than during convalescent phase and control. Mean LVFS was significantly lower in patients during acute phase than during convalescent phase and controls (p>0.001) Adrenomedulin was significantly higher in patients during acute phase than during convalescent phase and controls (p>0.001).Table 3 shows plasma Adrenomedulinin acute phase was negatively correlated with LVFS and positively correlated with ESR and ASOT) r =-0.46 & 0.52 p<0.01 & p<0.01 respectively.
|
|
|
4. Discussion
- In this study we examined the level of adrenomedulin in children with rheumatic fever during acute phase and convalescent phase and its relation to left ventricular function. We found the plasma ADM levels was significantly higher in children with ARF than control and in the acute phase more than in convalescent phases. This in agreement with Ednan et al, 2008 who was found that ADM level was significantly higher in adults with acute rheumatic fever this may be due to the fact that endogenous ADM may play an important role as acounter-regulatory hormone in states where there is pathological inflammatory reactions. Thus, increased levels of plasma ADM may also be compensatory against inflammatory reactions in ARF [14].Majority of patients with rheumatic carditis have normal LV systolic function. Congestive heart failure was more common in patients with a recurrence of carditis. All patients with congestive heart failure had hemodynamically significant valvular lesions, and majority had normal left ventricular systolic function. Patients with a reduced fractional shortening had significant valvular lesions. These observations are in agreement with recent reports [15, 16]. Suggesting that valvular disease, as opposed to myocardial dysfunction, is the mechanism of congestive heart failure in rheumatic carditis, it may represent the combined effects of elevated ventricular afterload, left ventricular enlargement and right ventricular volume overload. In this study, the plasma levels of BNP in patients with ARF in the acute and convalescent phases were found to be higher compared to healthy subjects.The elevated afterload and/or left ventricular enlargement seem to be the principal stimulus for BNP secretion in ARF patients. Adrenomedullin and brain natriuretic peptide are elevated in heart failure [17]. The negative correlation between ADM, BNP levels and EF in acute phase of disease suggests that such increased levels may be because of asymptomatic LV dysfunction. Plasma adrenomedullin is increased in several diseases [18]. Concentrations are raised incongestive heart failure and there is a weak inverse correlation with left ventricular ejection Fraction However, ADM was e still higher than those of control subjects in the convalescent phase of disease [19]. However, in patients with heart failure there is often coexisting left ventricular diastolic dysfunction, and this can be assessed readily and non-invasively by Doppler echocardiography [20]. Other vasodilator neuropeptides that are increased in heartfailure — such as atrial and brain natriuretic peptides—show a greater increase in patients with restrictive diastolic filling, which is the most severe form of diastolic dysfunction. Although we obtained the convalescent phase samples within 3 months after the diagnosis, most of patients (79.8%) had carditis and valvular lesions. Moreover, most of these patients may have sub-clinical inflammation in the convalescent phase of disease. Therefore, increased ADM levels in the convalescent phase maybe related to possible lasting effect of sub-clinical inflammation. Increased BNP levels in this phase may also be related to persistence of LV dysfunction [21].Study limitationsFurther prospective studies including the importance of ADM and BNP levels in the follow up and management of patients with ARF are needed. Although BNP and ADM levels were higher inthe acute and convalescent phase of the disease, whether these parameters may be used as follow-up markers like C-reactive protein and erythrocyte sedimentation rate in acute rheumatic fever should be investigated.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML