-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2017; 7(2): 61-66
doi:10.5923/j.ajmms.20170702.04

Relation between Vitamin D Deficiency Rickets and Atopic Dermatitis
Khaled Hassaan Awad1, Ismael Abd El Razek K. M. El-Lebedy1, Ahmad Saad Al Shemy1, Refaat Ragheb Mohamed2, Ali S. M.3
1Pediatric Department, Al-Azhar University, Assiut, Egypt
2Dermatology Department, Al-Azhar University, Assiut, Egypt
3Clinical Pathology Department, Al-Azhar University, Assiut, Egypt
Correspondence to: Khaled Hassaan Awad, Pediatric Department, Al-Azhar University, Assiut, Egypt.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

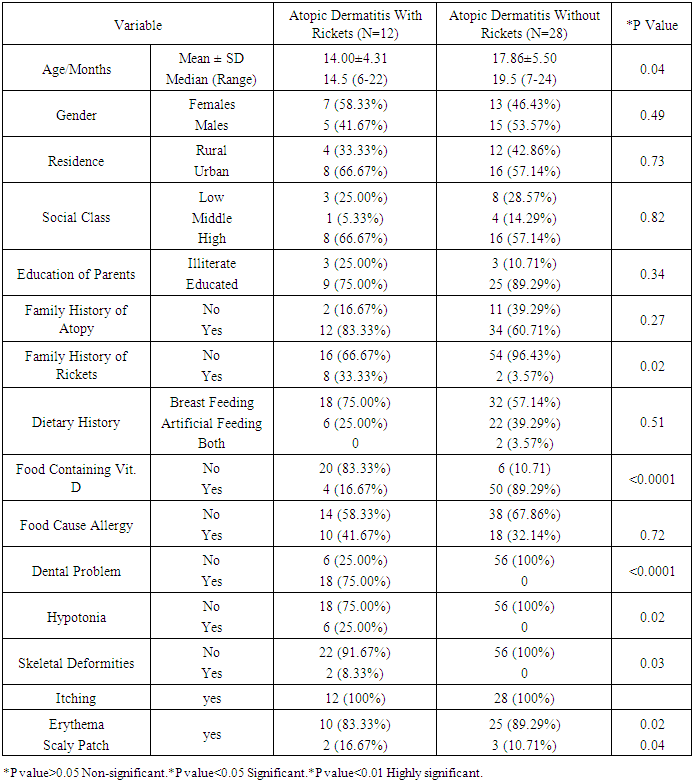
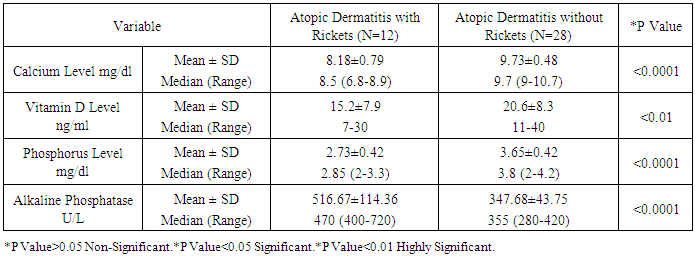
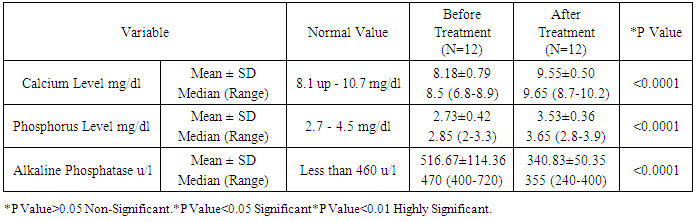
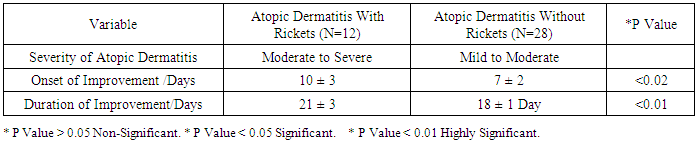
As the pathogenesis of atopic dermatitis involves a complex interplay of epidermal barrier dysfunction and dysregulated immune response, and vitamin D is involved in both processes, it is reasonable to expect that vitamin D could be associated with atopic dermatitis' risk or severity. Such association is suggested by epidemiological and experimental data. The aim of this work is to study the association between atopic dermatitis and vitamin D deficiency rickets among children from 6 months up to two years. Patients and methods: this study was conducted on 40 children aged from 6-24 month, suffering from atopic dermatitis. They were subjected to full history taking, clinical examination including: assessment of growth and development, manifestations of atopic dermatitis, manifestations of rickets and examination of other systems. Serum vitamin D level, serum calcium level, Serum phosphorus level, Alkaline phosphatase enzyme level and Plain x-ray on long bones were done. Patients with atopic dermatitis were divided into 2 groups: (1) Group I: Atopic dermatitis with Rickets. (2) Group II: Atopic dermatitis without Rickets.- Children with dyslipidemia and or malnutrition were excluded from the study. Vitamin D therapy was given to group 1 for two months in addition to treatment of AD for both groups. Follow up of both groups for two months was done by clinical and laboratory assessment. Results: A statistically significant difference were found between group 1 and group 2 as regard the age (p=0.04), weight (p=0.04), body mass index (p=0.006), length (p=0.003), length percentile (p=0.02), anterior fontanel (p=0.004) and its size (p=0.32), family history of rickets (p=0.02), food containing vitamin D (p<0.0001), dental problems (p<0.0001), hypotonia (p=0.02), itching (p<0.0001), skin lesions (p<0.0001), vitamin D level, calcium level (p<0.0001), phosphorus level (p<0.0001) and alkaline phosphatase level (p<0.0001). There was a non-statistically significant differences between group 1 and group 2 as regard gender (p=0.49), residence (p=0.73), social class (p=0.82), education of parents (p=0.34), dietary history (p=0.51), weight percentile (p=0.91), head circumference (p=0.63), head circumference percentile (p=0.09)and weight/length (p=0.09), food causing allergy (p=0.72), skeletal deformities (p=0.30), skin lesion (p=0.63) and skin lesion distribution (p=0.24). After treatment with vitamin D there was a statistically significant difference between group 1 (AD with rickets) and group 2 (AD without rickets) as regard onset of improvement (p<0.02), duration of improvement (p<0.01), itching (p<0.0001), skin lesions (p<0.0001) and anterior fontanel (p=0.004) and its size (p=0.32). Conclusions: children with atopic dermatitis and rickets have a lower vitamin D, Calcium and phosphorus levelsand higher alkaline phosphatase enzyme and moreatopic symptoms than those with atopic dermatitis without rickets. Also, children treated with vitamin D have lower duration of treatment and earlier onset of improvement more than those treated without vitamin D.
Keywords: Vitamin D, Rickets, Atopic dermatitis, Serum calcium
Cite this paper: Khaled Hassaan Awad, Ismael Abd El Razek K. M. El-Lebedy, Ahmad Saad Al Shemy, Refaat Ragheb Mohamed, Ali S. M., Relation between Vitamin D Deficiency Rickets and Atopic Dermatitis, American Journal of Medicine and Medical Sciences, Vol. 7 No. 2, 2017, pp. 61-66. doi: 10.5923/j.ajmms.20170702.04.
1. Introduction
- Atopic dermatitis (AD) is a chronic and relapsing inflammatory skin condition characterized by dry, itchy skin and immunologic hyper responsiveness to allergens [1]. AD affects 10% to 20% of children worldwide, consisting a serious public Health concern [2]. Severe AD adversely affects quality of life and may lead to significant morbidity due to skin infections and atopic comorbidity however ,other serious but less recognized AD complications may occur, including abnormalities in bone metabolism [3]. Vitamin D (VD) deficiency affects large number of people worldwide [4]. Extremely low level of vitamin D (<12nmol/L) are associated with development of rickets or osteomalacia, but insufficient levels (<75nmol/L) are linked to wide variety of non-skeletal diseases including infections and allergies [5]. Sun exposure is the most cost effective means of obtaining VD, because most VD in human is synthesized from precursors in the skin to VD3. Some foods such as oily fish also contain VD3 which metabolized in the liver and kidneys to produce 1,25 dihydroxy VD which is active hormone and act as a regulator of mineral hemostasis [4]. An association between VD deficiency and allergic diseases has been provoked [6]. Epidemiological data have shown a higher prevalence of allergic diseases including AD, food allergy and anaphylaxis at higher altitude, used as a proxy of sun exposure [5]. Lower vitamin D level also appear to correlate with AD severity which suggests a potential role VD in the pathogenesis of AD [7]. VD has widespread effects on the immune system and skin integrity VD been shown to include tolerogenic dendritic cells and regulatory T-cells and is able to suppress T cells mediated immunity [8]. VD also modulate innate immunity, particularly boosting immunity to Staphylococcus aurous, the major skin pathogen of patients with AD [9]. In addition VD is relevant for epidermal differentiation and skin barrier permeability, essential factors that are altered in patients with AD [10]. The aim of this work is to study the association between atopic dermatitis and vitamin D deficiency rickets among children from 6 months up to two years and the effect of treatment with vitamin D.
2. Patients and Methods
- This study was carried out from June 2015 to February 2016 in Pediatric and Dermatology outpatient clinic at Sohag Teaching Hospital, Sohag city, Egypt; and Al-Azhar University Hospital, Assiut, Egypt. It was conducted on40 child renaged from 6-24 month and was approved by the Institutional Scientific and Ethical Committee, and written informed consents were obtained from the parents. All members of the study were subjected to the following: Full history taking including socio-demographic data, family history of atopic dermatitis, dietary and developmental histories. Clinical examination: assessment of growth and development, manifestations of atopic dermatitis, manifestations of rickets and examination of other systems. Serum vitamin D, serum calcium, phosphorus and alkaline phosphatase enzyme level were done for all patients. Plain x-ray on long bones. All cases suffered from Atopic Dermatitis were divided into two groups: (1) Group I: Atopic dermatitis with Rickets. (2) Group II: Atopic dermatitis without Rickets. Exclusion criteria: children with other skin allergic dermatitis, other types of rickets, children below 6 months or older than 2 years, malnourished children and those having dyslipidemia and children receiving vitamin D. Vitamin D therapy was given to group 1 for two months(2000-5000 international units (IU) of vitamin D /day for infants or intramuscular injection of vitamin D at doses of 150,000–600,000 IU/dose, with repeated dosing as needed+ calcium gluconate 40 mg /kg/day. and treatment of tetany if present. In addition to treatment of AD for both groups. Follow up of both groups for two months was done for clinical and laboratory assessment. Sample collection: Three ml venous blood samples were drawn from all patients under aseptic condition in a sterile plain vacutainer for investigations, serum for sample analysis was obtained following centrifugation of whole blood after complete clot formation has taken place at 3000 rpm for 5 min, serum was stored at -20°C: Methods: 1- The principle for assay of vitamin D: The 25 OH Vitamin D was measured by VIDAS (BioMerieux, France, 30463) according to the manufacturer's instructions. Briefly, the sample was mixed with pre-treatment reagent to separate vitamin D from its binding protein. The pre-treated sample was collected and transferred into the well that contains an alkaline phosphatase (ALP)- labeled anti-vitamin D antibody (conjugate). The vitamin D antigen present in the sample and the vitamin D antigen coating the interior of the Solid Phase Receptacle (SPR) compete for binding sites on the anti-vitamin D antibody-ALP conjugate. The substrate (4-Methyl- umbelliferyl phosphate) is cycled in and out of the SPR. The conjugate enzyme catalyzes the hydrolysis of this substrate into a fluorescent product (4-Methyl-umbelliferone), the fluorescence of which was measured at 450 nm. The intensity of the fluorescence is inversely proportional to the concentration of vitamin D antigen present in the sample. At the end of the assay, results are automatically calculated by the instrument in relation to the calibration curve stored in memory [11]. 2- The principle for assay of calcium: Calcium was assayed by O-cresolphthaleincomplexone method (Randox, United Kingdom, CA 590), as calcium ions form aviolet complex with O-cresolphthaleincomplexone in an alkaline medium, the color intensity of the complex formed is directly proportional to the calcium concentration. It is determined by measuring the increase in absorbance at 578 nm [12]. 3- The principle for assay of Phosphorus (Randox, United Kingdom, PH 1016). Inorganic phosphorous reacts with ammonium molybdate in the presence of sulphuric acid to form a phosphomolybdate complex which is measured at 340 nm [13]. 4- The principle for assay of Alkaline phosphatase (Spinreact, Spain, 41240). ALP catalyses the hydrolysis of p-nitrophenyl phosphate at pH 10.4, liberating p-nitrophenol and phosphate, the rate of p-nitrophenol formation, measured photometrically, is proportional to the catalytic concentration of alkaline phosphatase present in the sample.Statistical analysis: data was analyzed using STATA intercooled version 12.1. Quantitative data was represented as mean, standard deviation, median and range. Data was analyzed using student t-test to compare means of two groups and paired t test to compare results before and after treatment. When the data was not normally distributed Mann-Whitney and Wilcoxon test were used. Qualitative data was presented as number and percentage and compared using either Chi square test or fisher exact test. Graphs were produced by using Excel or STATA program. P value was considered significant if it was less than 0.05. For all these tests, the level of significance (P-value) can be explained as:*Non- significant P > 0.05.*Significant P < 0.05.*Highly significant P < 0.01.
3. Results
|
|
|
|
4. Discussion
- In the present study we found a statistically significant difference between group 1 (AD with rickets) and group 2 (AD without rickets) as regard the age (p value =0.04). There was a non-statistically significant difference between group 1 (AD with rickets) and group 2 (AD without rickets) as regard gender (p value = 0.49), residence (p value = 0.73), social class (p value = 0.82) and education of parents (p value = 0.34). These results were in agreement with Williams and Flohr, 2006, [14] who reported that in 85% of cases, atopic dermatitis occurs in the first year of life; in 95% of cases, it occurs before age 5 years. For gender females are slightly more likely than males to get AD, also AD seems more common in higher social classes according to Chan M, 2002, [15]. In the present study we found a highly statistically significant difference between group 1 (AD with rickets) and group 2 (AD without rickets) as regard dental problems (p value<0.0001) and food containing vitamin D (p value < 0.0001). These results were in agreement with Tom, et al., 2000, [16], who reported that mean (± SD) daily dietary calcium intake was low in both children with rickets and control children (217 ± 88 mg and 214 ± 77 mg, respectively; P value = 0.64). Also there was a statistically significant difference between group 1 (AD with rickets) and group 2 (AD without rickets) as regard family history of rickets (p value=0.02) and hypotonia p value = 0.02. These results were in agreement with Thatcher, et al., 2011, [17], who reported that children with rickets had a greater proportion of first-degree relatives with a history of rickets (14.6% vs 3.1%; P <.001). There was a non-statistically significant difference between group 1 (AD with rickets) and group 2 (AD without rickets) as regard family history of atopy (p value=0.27), dietary history p value = 0.51, food causing allergy (p value=0.72) and skeletal deformities (table 2) and p value=0.30. In the present study we found a highly statistically significant difference between group 1 (AD with rickets) and group 2 (AD without rickets) as regard length (p value =0.003), and body mass index (p value=0.006). These results were in agreement with, Kao, et al., 1991, [18], who reported that children suffering from rickets with Surveys in Taiwan have observed increases in their average body weight and body mass index (BMI), as well as the prevalence of obesity. There was a statistically significant difference between group 1 (AD with rickets) and group 2 (AD without rickets) as regard weight (p value = 0.04), length percentile (p value = 0.02) and actual Wt./L / expected wt./L and p value = 0.02. These results were in agreement with White, et al., 1995 [19], who reported that from measurements of Tayside children (N = 34,533) centile charts were constructed for BMI (wt/ht2) and BMI / centiles from the raw data of height and weight, using Cole's LMS method for normalized growth standards. These data were compared with the only available European BMI charts. There was a non-statistically significant difference between group 1 (AD with rickets) and group 2 (AD without rickets) as regard weight percentile (p value = 0.91), head circumference and p value = 0.63, head circumference percentile (p value = 0.09) and weight/length (p value = 0.09). In the present study we found a highly statistically significant difference between group 1 (AD with rickets) and group 2 (AD without rickets) as regard presence of anterior fontanel opening (p value = 0.004). This was in agreement with Elidrissy, 2000, [20], who reported that Fifty-four cases of clinically diagnosed rickets, and subsequently confirmed radiologically, were compared to 28 controls. Half had signs of the disease in limbs, skull (anterior fontanel opening), and chest. There was a non-statistically significant difference between group 1 (AD with rickets) and group 2 (AD without rickets) as regard type of skin lesion (p value =0.63), skin lesion distribution (p value=0.24) and size of anterior fontanel (p value = 0.32). In the present study we found a highly statistically significant difference between group 1 (AD with rickets) and group 2 (AD without rickets) as regardvitamin D levels, (p value 0.01), (calcium level (p value < 0.0001), phosphorus level (p value<0.0001) and alkaline phosphatase level (p value<0.0001).Vitamin D deficiency may present with hypocalcaemia, low phosphorus level [21]. In the present study we found a highly statistically significant difference between group 1 (AD with rickets) before and after treatment as regard itching (p value < 0.0001) and skin lesions (p value < 0.0001). These results were in agreement with Sewon Kang, et al., 2001, [22] who reported that treatment of AD lesions by tacrolimus ointment (non-steroidal topical immunomodulation) for 255 children, 1 to 15 years of age, with moderate to severe atopic dermatitis applied 0.1% tacrolimus ointment twice daily for up to 12 months to assess long-term safety and efficacy. Substantial improvements in the signs and symptoms of atopic dermatitis, and the patient's or parent's assessment of pruritus were observed during the first week of treatment and were maintained throughout the study. Occurrence of these symptoms decreased after the first few days of treatment. There was a statistically significant difference between group 1 (AD with rickets) before and after treatment as regard presence of anterior fontanel opening (p value = 0.03) and size of anterior fontanel, p value = 0.02. In the present study we found a highly statistically significant difference between group 1 (AD with rickets) before and after treatment as regard calcium level, phosphorus level and alkaline phosphatase level (p value <0.0001). These results were in agreement with Pettifor, 2004, [23] who reported that treatment of 47 Jordanianinfants with nutritional rickets with one injection of 600,000 IU of vitamin D resulted in normalization of serumcalcium and phosphate levels, and a significant decrement inalkaline phosphatase within 3 weeks of therapy. Vitamin Dresponsible for maintaining calcium and phosphorus homeostasis. As the increased expression of the calcium channel permits more calcium to enter the cell, where the vitamin D–dependent calcium-binding protein helps calcium’s translocation into the bloodstream. 1,25(OH)2D also enhances phosphorus absorption in the small intestine [24]. In the present study we found astatistically significant difference between group 1 (AD with rickets) and group 2 (AD without rickets) as regard onset of improvement (p value <0.02), duration of improvement and severity of atopic dermatitis. These results were in agreement with Lee, et al., 2013, [25] who study AD in 157 patients, 73.3% of which between the ages of 0–15, showed that mean serum levels of vitamin D were significantly higher in patients with mild AD compared to those with moderate or severe AD. In this study we found a statistically significant difference between group 1 (AD with rickets) and group 2 (AD without rickets) as regarding improvement of atopy rash and itching in children with AD and rickets after treatment with vitamin D beside treatment of atopy. Improvement of rash and itching among group (2) AD without rickets occurs rapidly than improvement of AD among group (1) AD with rickets (p value<0.01). These results were in agreement with Carlos, et al., 2014, [26] who reported that daily treatment with a vitamin D supplement significantly reduced the symptoms of winter-related atopic dermatitis in more than 100 Mongolian schoolchildren, Peroni, et al., 2011, [27] Evaluated the relationship between vitamin D and AD severity. The study included 37 children with AD. Serum levels of 25(OH)D were higher in patients with mild AD compared to those with moderate or severe cases (p value<0.05). Data suggest that vitamin D deficiency may be related to the severity of AD. Similar results were obtained by El Taieb, et al., 2013, [28], who compared 29 AD children to a control group of 30 healthy individuals, and Wang, et al., 2014, [29], who considered 498 Hong Kong Chinese children affected by AD and compared them to 328 controls. Overall these data seem to indicate that vitamin D deficiency is related to the severity of AD. There are, however, many controversies. Despite the above evidences, several authors have had opposing results, Bäck, et al., 2009, [30] observed that higher intake of vitamin D during the first year of life was correlated with increased risk of eczema at age six. 123 children 'were investigated through a postal questionnaire looking for the cumulative incidence of AD, allergic rhinitis, or asthma at 6 years of age. Regardless of family history of atopy, AD was more prevalent in those with the highest intake of vitamin D. Through a nationwide cross-sectional survey on 9838 German children with eczema, Heimbeck, et al., 2013, [31] found a significantly reduced risk of eczema for the lowest vitamin D serum quartile compared to the reference quartile in a multivariate analysis. Chiu , et al., 2013, [32] evaluated 94 children of 1 to 16 years old living in urban Milwaukee (USA), finding no statistically significant association between vitamin D levels and AD severity. Further, children with mild AD had serum levels of 25(OH)D lower than patients with moderate and severe diseases, although this difference was not statistically significant.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML